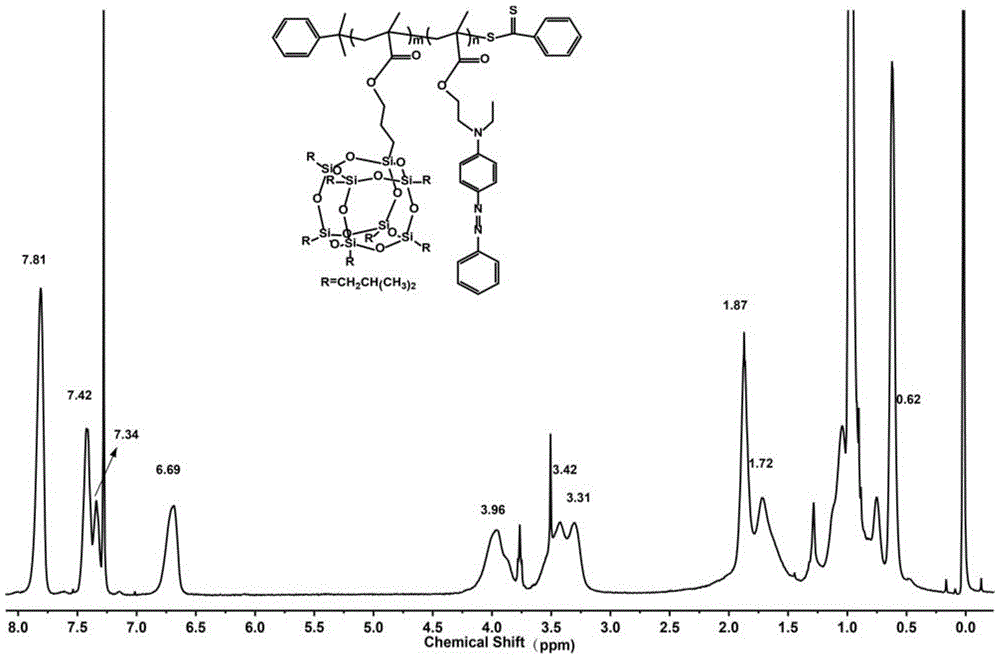
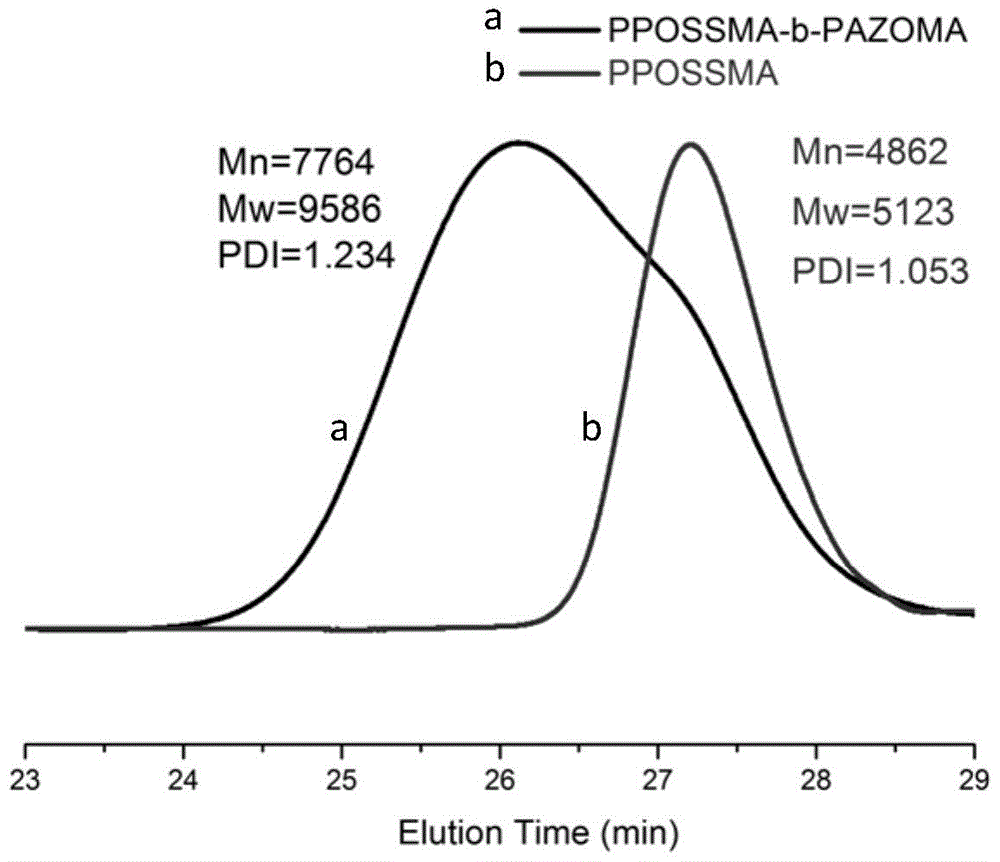
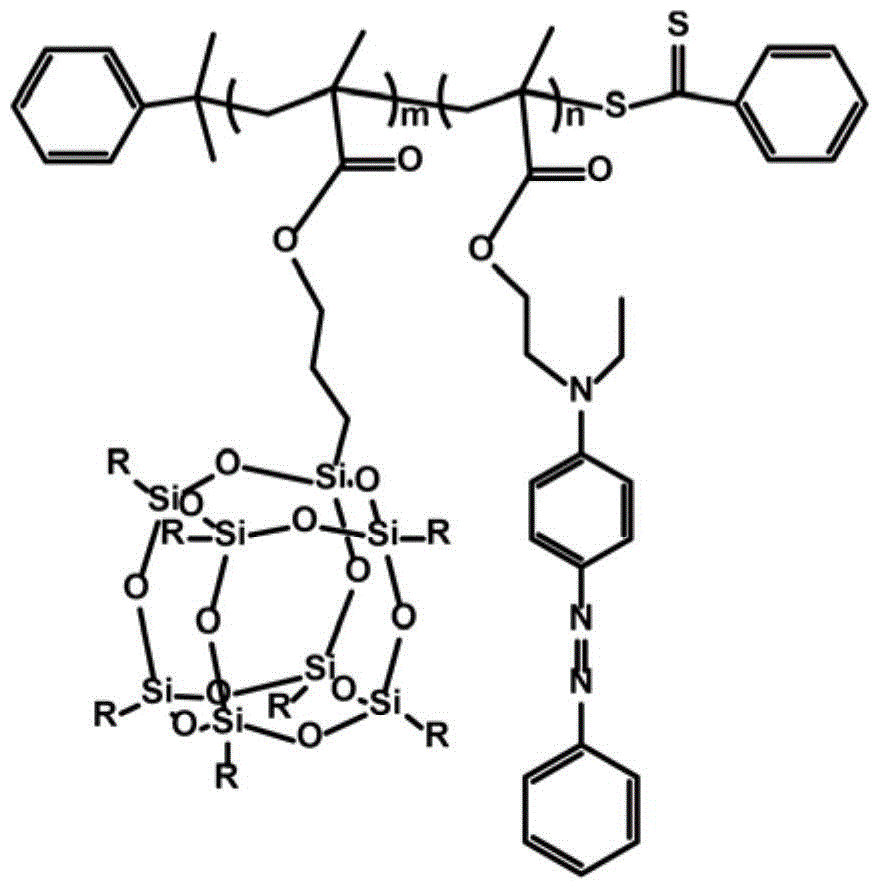
Silicon nitrogen containing flame retardant type polymer dye and preparation method thereof
A polymer and flame-retardant technology, applied in the field of silicon-nitrogen-containing flame-retardant polymer dyes and their preparation, can solve the problem of high requirements for synthesis equipment, high temperature and high pressure reaction, and difficulty in controlling the content of azobenzene flame retardant groups, etc. problems, to reduce migration, improve flame retardancy and anti-droplet properties, and achieve the effect of good adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) PPOSSMA m Synthesis of Macromolecular Chain Transfer Agents
[0022] Methacryloxypropyl heptaisobutyl polyhedral oligomeric silsesquioxane POSSMA (5.2808g), cumyl dithiobenzoate CDB (0.1524g) and azobisisobutyronitrile AIBN (0.0274g ) was dissolved in 8.0ml tetrahydrofuran, after continuous freeze-thaw degassing for 5 times, the RAFT polymerization reaction was carried out under the protection of argon, and then the reaction was quenched by liquid nitrogen, and methanol: ethyl acetate was 8: 1 as the precipitant repeatedly. Precipitate 3 times to get the product PPOSSMA m Macromolecular chain transfer agent;
[0023] 2) PPOSSMA m -b-PAZOMA n Synthesis of diblocks
[0024] Take step 1) synthesized PPOSSMA m Macromolecular chain transfer agent (1.1300g) and 4-{[(2-methacryloyloxy)ethyl]ethylamino} azobenzene AZOMA (1.0122g) and azobisisobutyronitrile (0.0050g) Dissolve in 3.0ml tetrahydrofuran, conduct RAFT polymerization reaction under the protection of argon a...
Embodiment 2
[0028] 1) PPOSSMA m Synthesis of Macromolecular Chain Transfer Agents
[0029] Methacryloxypropyl heptaisobutyl polyhedral oligomeric silsesquioxane POSSMA (2.8290g), cumyl dithiobenzoate CDB (0.0454g) and azobisisobutyronitrile AIBN (0.0082g ) was dissolved in 5.0ml tetrahydrofuran, after continuous freeze-thaw degassing for 5 times, RAFT polymerization was carried out under the protection of argon, and then quenched with liquid nitrogen to stop the reaction, and methanol:ethyl acetate was 7:1 as the precipitating agent repeatedly Precipitate 3 times to get the product PPOSSMA m Macromolecular chain transfer agent;
[0030] 2) PPOSSMA m -b-PAZOMA n Synthesis of diblocks
[0031] Take step 1) synthesized PPOSSMA m Macromolecular chain transfer agent (1.6950g) and 4-{[(2-methacryloyloxy)ethyl]ethylamino} azobenzene AZOMA (1.0122g) and azobisisobutyronitrile (0.0050g) Dissolve in 3.0ml tetrahydrofuran, conduct RAFT polymerization reaction under the protection of argon aft...
Embodiment 3
[0033] 1) PPOSSMA m Synthesis of Macromolecular Chain Transfer Agents
[0034] Methacryloxypropyl heptaisobutyl polyhedral oligomeric silsesquioxane POSSMA (2.6404g), cumyl dithiobenzoate CDB (0.0762g) and azobisisobutyronitrile AIBN (0.0137g ) was dissolved in 5.0ml tetrahydrofuran, and after continuous freeze-thaw degassing for 5 times, RAFT polymerization was carried out under the protection of argon, and then quenched with liquid nitrogen to stop the reaction, and methanol:ethyl acetate was 8:1 as the precipitant repeatedly. Precipitate 3 times to get the product PPOSSMA m Macromolecular chain transfer agent;
[0035] 2) PPOSSMA m -b-PAZOMA n Synthesis of diblocks
[0036] Take step 1) synthesized PPOSSMA m Macromolecular chain transfer agent (1.3560g) and 4-{[(2-methacryloyloxy)ethyl]ethylamino} azobenzene AZOMA (1.2164g) and azobisisobutyronitrile (0.0059g) Dissolve in 3.5ml tetrahydrofuran, conduct RAFT polymerization reaction under the protection of argon after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com