Xylanase gene derived from streptomyces chartreusis and xylanase thereof
A technology of xylanase, SEQ-2, which is applied in the directions of genetic engineering, plant genetic improvement, and microorganism-based methods, and can solve problems restricting the application of xylanase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Gene Cloning of the Xylanase XynA of Streptomyceschartreusis L1105
[0035] 1.1 Genomic DNA extraction
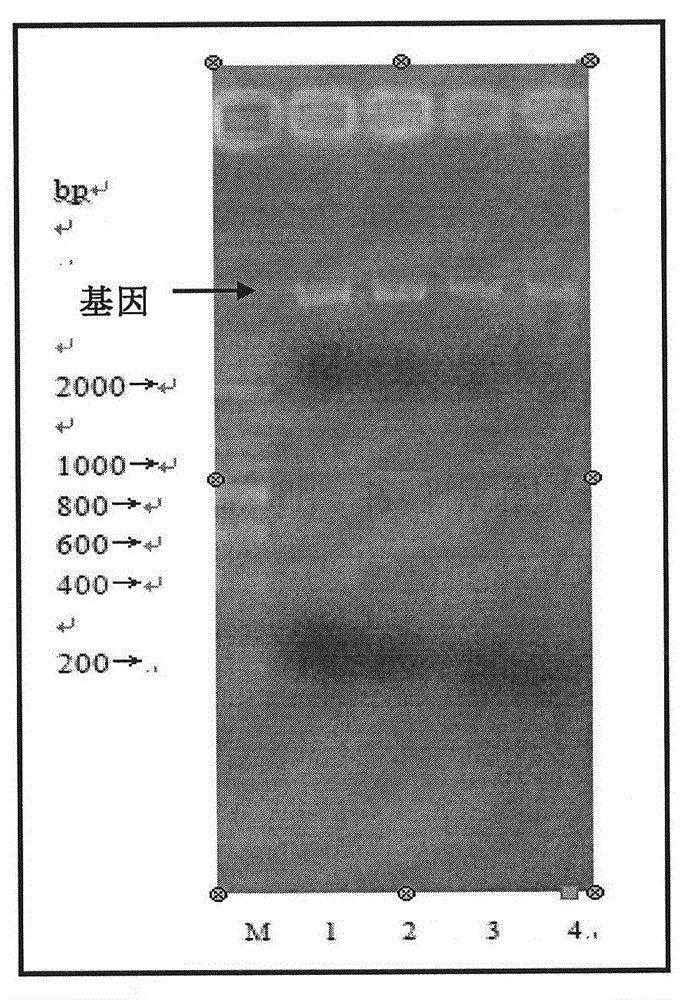
[0036] Inoculate Streptomyces elixirs L1105 in LB liquid medium, culture at 30°C, 150rpm for 72h, draw 1.5mL of the bacteria solution, put it in a 2mL centrifuge tube and centrifuge at 10000×g for 5min at room temperature to obtain the bacteria. Bacterial genome was extracted to obtain total xylanase genome DNA ( figure 1 ).
[0037] 1.2 Design of degenerate primers
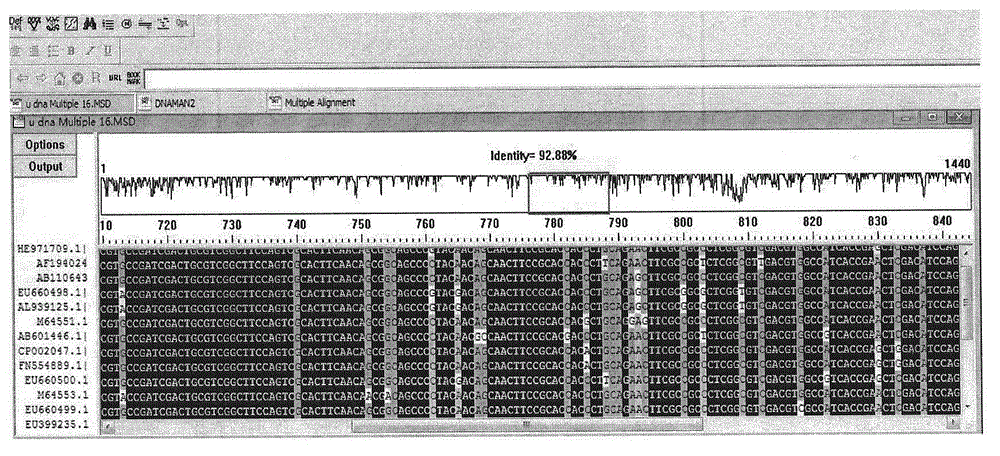
[0038] In the NCBI protein database (http: / / www.ncbi.nlm.nih.gov / ), enter the 15 amino acid sequences of the N-terminal of xylanase ASTLGAAAAEKGRYF (Ala-Ser-Thr-Leu-Gly-Ala-Ala-Ala- Ala-Glu-Lys-Gly-Arg-Tyr-Phe) was compared by Blastn, and 10 xylanases encoded by Streptomyces with high homology to the xylanase gene of Streptomyces elixirs L1105 were obtained Design primers in the high homology region of the gene sequence. The conserved sequence region ( figure 2 ).
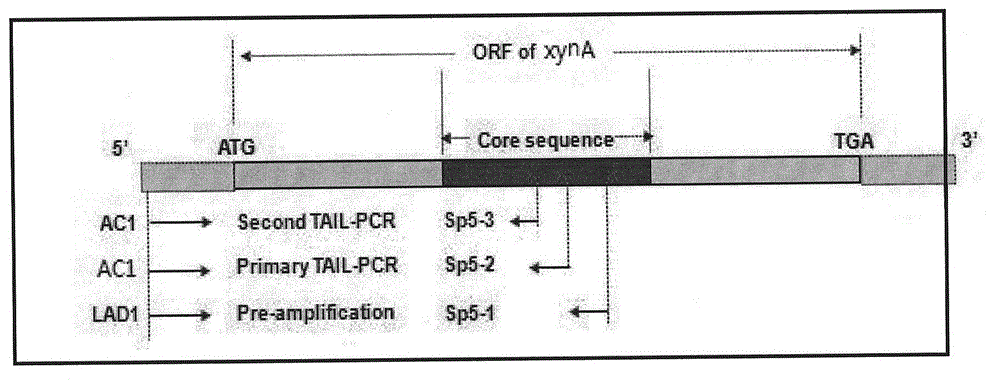
[0039] 1.3PCR ampl...
Embodiment 2
[0059] Embodiment 2: Analysis of recombinant xylanase XynA protein
[0060] 2.1 Construction of pET28a(+) expression vector:
[0061] 2.1.1 PCR amplification gene xynA
[0062] Primers were designed according to xynA, considering the multiple cloning site (MCS) of the expression vector and the sequence of xynA, and restriction sites EcoRI and XhoI were introduced at both ends of xynA. Refer to Table 2.1 for the PCR reaction system. The PCR cycling conditions were: 94°C, 4min; 94°C, 30s; 68°C, 30s; 72°C, 90s; a total of 30 cycles, with a final extension of 10min at 72°C. PCR products were recovered by gel cutting. The primers were synthesized by Beijing Aoke Dingsheng Company, and the primers are listed in Table 3.
[0063] Table 3 Primers for cloning xynA
[0064] Table3PrimersforxynA
[0065]
[0066] 2.1.2 Construction of recombinant plasmid pET28a-xyn4
[0067] The expression vector pET28a and xynA with restriction sites were digested with EcoRI and XhoI, respecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com