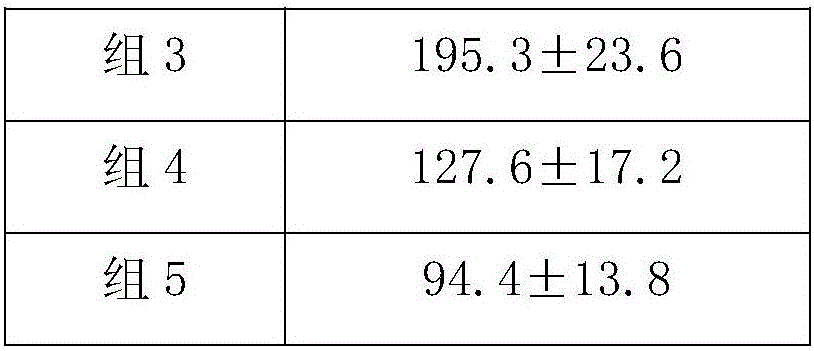The ctl epitope of hepatitis B virus core antigen and its related application
A hepatitis B virus and epitope peptide technology, applied in the direction of viruses, viral peptides, viruses/phages, etc., can solve problems such as inability to cure hepatitis B or other complications, and inability to effectively block liver fibrosis and sclerosis. Fibrosis and sclerosis, improvement of clinical symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Study on Antiviral Activity of Dendritic Cells Loaded with Epitope Peptides
[0017] Preparation of epitope peptide-loaded dendritic cells: extract mouse femoral bone marrow cells under sterile conditions, put them into PRMI1640 culture medium (containing 10% fetal bovine serum, 0.1ml penicillin-streptomycin) and mix well by pipetting for 3 hours Then take the adherent cells and add IL-4 (final concentration is 500U / ml) and GM-CSF (final concentration is 1000U / ml), at 37°C, 5% CO 2 cultured in an incubator. Half of the medium was replaced every 3 days, and GM-CSF and IL-4 were added at the same time to induce the generation of dendritic cells. After culturing for 10 days, add a final concentration of 0.1mg / L epitope peptide and 1×10 6 Dendritic cells per L were co-incubated at 37°C for 24 hours, and the activated dendritic cells were collected.
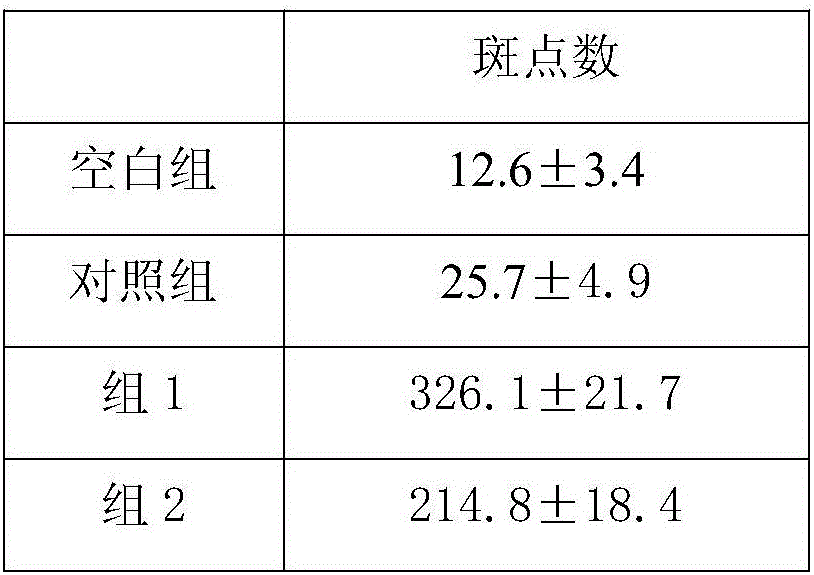
[0018] Female HBV transgenic Babl / c mice aged 6-8 weeks were randomly divided into experimental group, control g...
Embodiment 2
[0026] Example 2 Antiviral Clinical Research of Dendritic Cells Loaded with Epitope Peptides
[0027] Clinically collected 200 patients who were HBeAg positive and had not received antiviral or immunomodulatory drug treatment for 6 months before administration. After testing, its HBV-DNA≥1000copies / mL (ALT≥40IU, ALT<40IU).
[0028] Preparation of dendritic cells loaded with epitope peptides: The peripheral blood mononuclear cells of the patient were isolated by leukapheresis, and the purity of CD14+ cells was up to 94%. After being treated with GM-CSF and IL-4, the cells became obvious dendrites. On the 10th day of culture, most of the cells expressed co-stimulatory molecule markers CD86, CD40, CD80 and HLA-DR, MHC-I molecules. Immediately add the epitope peptide of final concentration 0.1mg / L (epitope peptide sequence is with embodiment 1) and 1 * 10 6 Dendritic cells / L were co-incubated at 37°C for 24 hours, and the activated dendritic cells were collected.
[0029] Intra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com