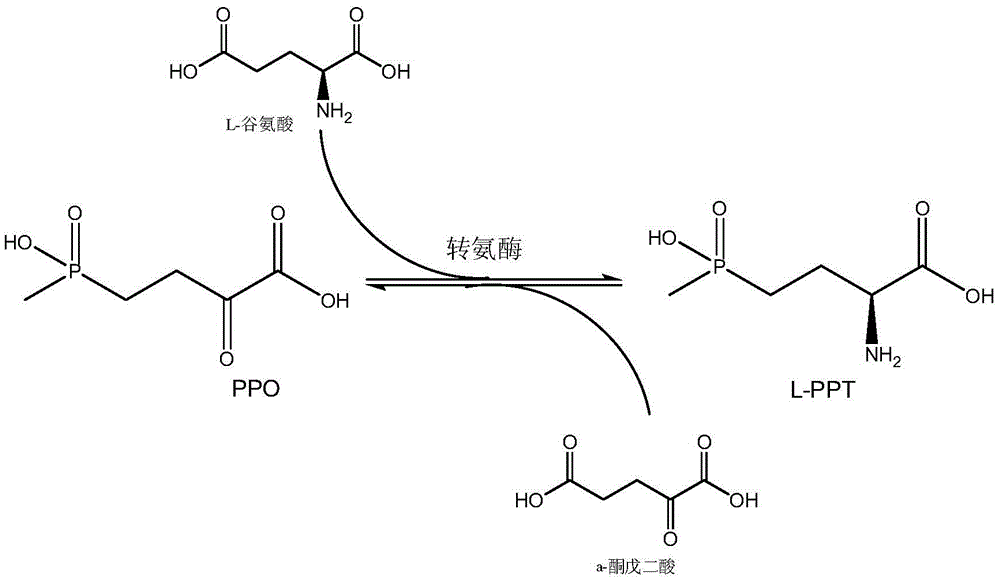
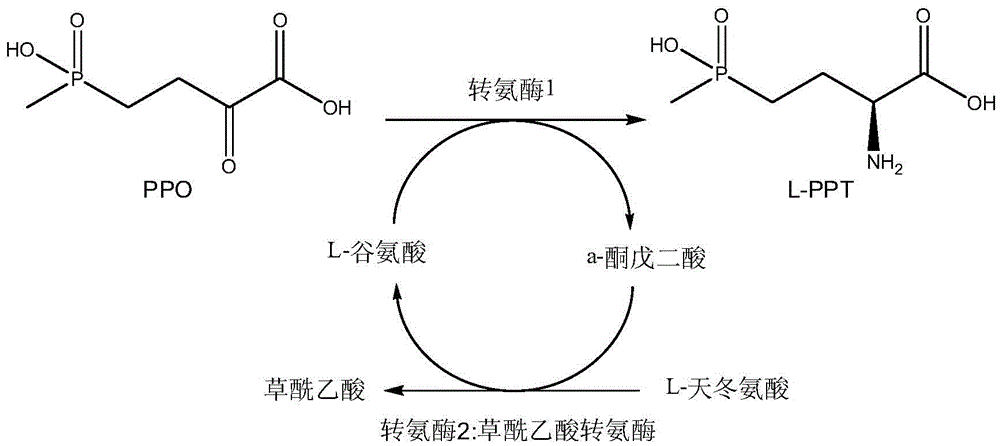
Production method of L-glufosinate
A production method, glufosinate-ammonium technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve problems such as low process efficiency, achieve the effects of low environmental protection pressure, easy availability of raw materials, and simplified refining process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Cloning transaminase genes from the genomes of Escherichia coli E.coliK12W3110, Bacillus subtilis168 and Bacillus megaterium YYBM1 respectively, and designing corresponding transaminase genes according to the corresponding genomic DNA sequences (GenBank accession numbers are CP012868.1, CP010052.1 and CP001982.1, respectively). PCR upstream primers and downstream primers.
[0050] Primers for transaminases derived from E.coli:
[0051] EC-F sequence: 5'-CCG GAATTC ATGAGCAACAATGAATTCCATC-3' (EcoRI)
[0052] EC-R sequence: 5'-CCG CTCGAG TTAATCGCTCAGCGCATCC-3'(XholI)
[0053] Primers for transaminases derived from Bacillus Subtilis:
[0054] BS-F sequence: 5'-CCC GAGCTC ATGAGTCAAAACAACAGCAAGCATCA-3'(SacI)
[0055] BS-R sequence: 5'-CCC AAGCTT TTAAGCTCGCAGGCCCGCCT-3' (HindIII)
[0056] Primers for transaminases from Bacillusmagaterium:
[0057] BM-F sequence: 5'-CGC GGATCC ATGAGTCAAACTTTTAGCAA-3' (BamHI)
[0058] BM-R sequence: 5'-CCC AAGCTT TTACACTTCAA...
Embodiment 2
[0074] 1. The cultivation of microorganisms
[0075] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0076] The genetically engineered bacteria E.coliBL21(DE3) containing the transaminase gene was inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 500mL fresh LB liquid medium also containing 50μg / mlKan, shake culture at 37°C until OD 600 When it reaches about 0.8, add IPTG to its concentration of 0.3mM, and induce culture at 28°C for 20h. After the cultivation, the culture solution was centrifuged at 10,000 rpm for 10 min, the supernatant was discarded, the bacteria were collected, and stored in a -70°C ultra-low temperature refrigerator until use.
[0077] 2. Preparation of crude enzyme solution
[0078] The thalli collected after the cultivation was finished were ...
Embodiment 3
[0080]Quantitatively weigh PPO, alanine and pyridoxal phosphate, mix them in a beaker, adjust the pH of the solution to 7.5 with 30% ammonia water, add it to a volumetric flask, and dilute to volume with deionized water to obtain a final concentration of PPO of 60 mM and alanine The final concentration of acid is 180mM, and the final concentration of pyridoxal phosphate is 1mM.
[0081] Cultivate the genetically engineered bacteria capable of expressing transaminase shown in SEQ ID NO.1 (derived from Escherichia coli E.coliK12W3110), take 1mL of the culture solution, centrifuge at 10000rpm for 10min, discard the supernatant to obtain 5mg of cells; add it to 1mL of the above-mentioned mixed solution, Resuspended and reacted in a metal bath shaking reactor at 37°C for 24 hours.
[0082] After the reaction was completed, HPLC detection was carried out, and L-glufosinate-ammonium was generated, but no obvious PPO peak was found, indicating that the conversion rate of PPO reached 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com