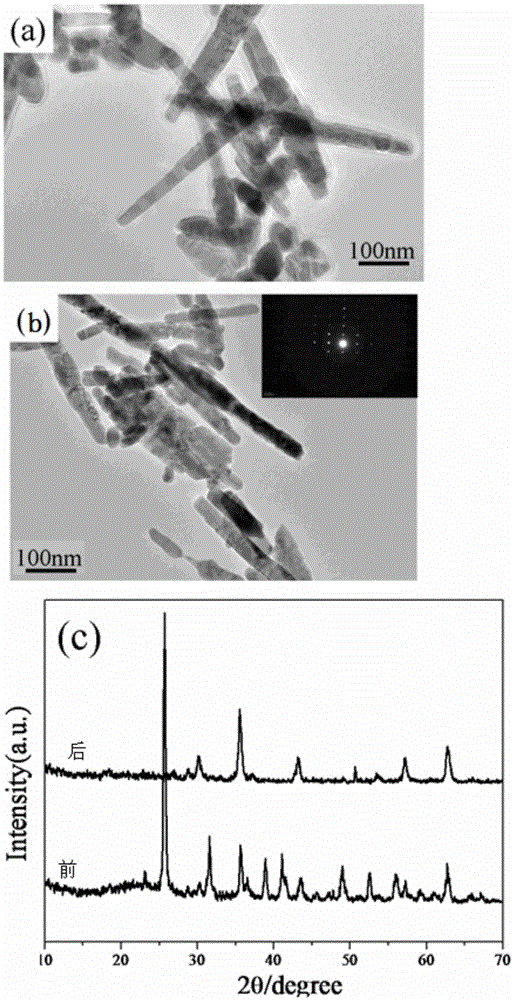
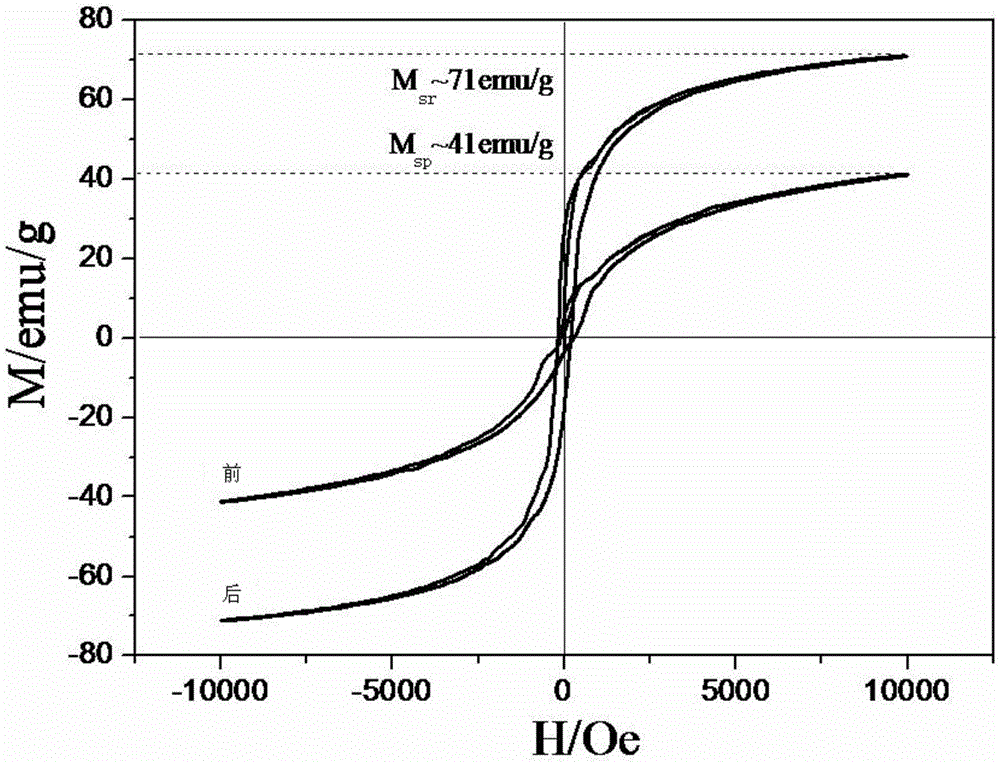
Method for macroscopic preparation of Fe3O4 nanorods
A nanorod, fe3o4caso4 technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of low product purity and difficulty in large-scale production, and achieve simple process, easy control of conditions, good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 (Fe 2 SO 4 ·7H 2 O and CaSO 4 2H 2 O weight ratio is 2:1)
[0025] Step 1, Preparation of α-FeOOH / CaSO 4 2H 2 O precursor
[0026] (1) Add 2g of Fe 2 SO 4 ·7H 2 O was dissolved in 100 mL of distilled water, and magnetically stirred for 2 minutes at room temperature to fully dissolve it to obtain solution a.
[0027] (2) Add 1g of CaSO 4 2H 2 O solution was slowly added to solution a, and magnetic stirring was continued for 3 minutes to obtain brownish red suspension b.
[0028] (3) A small amount of ammonia water was added dropwise to adjust the pH value of the suspension b to 10 to obtain a dark brown solution c.
[0029] (4) Filter solution c, and then dry it in an oven at 100°C for 2 hours to obtain α-FeOOH / CaSO 4 2H 2 O precursor.
[0030] Step 2, preparation of Fe by solid phase reduction reaction 3 o 4 CaSO 4 , post-processing to get Fe 3 o 4 Nano stave
[0031] (1) A certain amount of α-FeOOH / CaSO 4 2H 2 The O precursor was put i...
Embodiment 2
[0037] Example 2 (Fe 2 SO 4 ·7H 2 O and CaSO 4 2H 2 O weight ratio is 1:1)
[0038] Step 1, Preparation of α-FeOOH / CaSO 4 2H 2 O precursor
[0039] (1) Add 2g of Fe 2 SO 4 ·7H 2 O was dissolved in 100 mL of distilled water, and magnetically stirred for 3 minutes at room temperature to fully dissolve it to obtain solution a.
[0040] (2) Add 2g of CaSO 4 2H 2 O solution was slowly added to solution a, and magnetic stirring was continued for 2 minutes to obtain brownish red suspension b.
[0041] (3) A small amount of ammonia water was added dropwise to adjust the pH value of the suspension b to 7 to obtain a dark brown solution c.
[0042] (4) Filter solution c, and then dry in an oven at 60°C for 6 hours to obtain α-FeOOH / CaSO 4 2H 2 O precursor.
[0043] Step 2, preparation of Fe by solid phase reduction reaction 3 o 4 CaSO 4 , post-processing to get Fe 3 o 4 Nano stave
[0044] (1) A certain amount of α-FeOOH / CaSO 4 2H 2 The O precursor was put into a...
Embodiment 3
[0047] Embodiment 3 (Fe 2 SO 4 ·7H 2 O and CaSO 4 2H 2 O weight ratio is 1:2)
[0048] Step 1, Preparation of α-FeOOH / CaSO 4 2H 2 O precursor
[0049] (1) Add 1g of Fe 2 SO 4 ·7H 2 O was dissolved in 100 mL of distilled water, and magnetically stirred at room temperature for 1 minute to fully dissolve to obtain solution a.
[0050] (2) Add 2g of CaSO 4 2H 2 O solution was slowly added to solution a, and magnetic stirring was continued for 3 minutes to obtain brownish red suspension b.
[0051] (3) A small amount of ammonia water was added dropwise to adjust the pH value of the suspension b to 12 to obtain a dark brown solution c.
[0052] (4) Filter solution c, and then dry it in an oven at 80°C for 6 hours to obtain α-FeOOH / CaSO 4 2H 2 O precursor.
[0053] Step 2, preparation of Fe by solid phase reduction reaction 3 o 4 CaSO 4 , post-processing to get Fe 3 o 4 Nano stave
[0054] (1) A certain amount of α-FeOOH / CaSO 4 2H 2 The O precursor was put int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com