Biodegradable photocurable medical adhesive and preparation and application thereof
An adhesive and light-curing technology, applied in the fields of application, surgical adhesives, medical science, etc., can solve the problems of rapid flushing, easy water swelling, cell damage, etc., and achieve convenient sizing and high bonding strength , good bonding performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
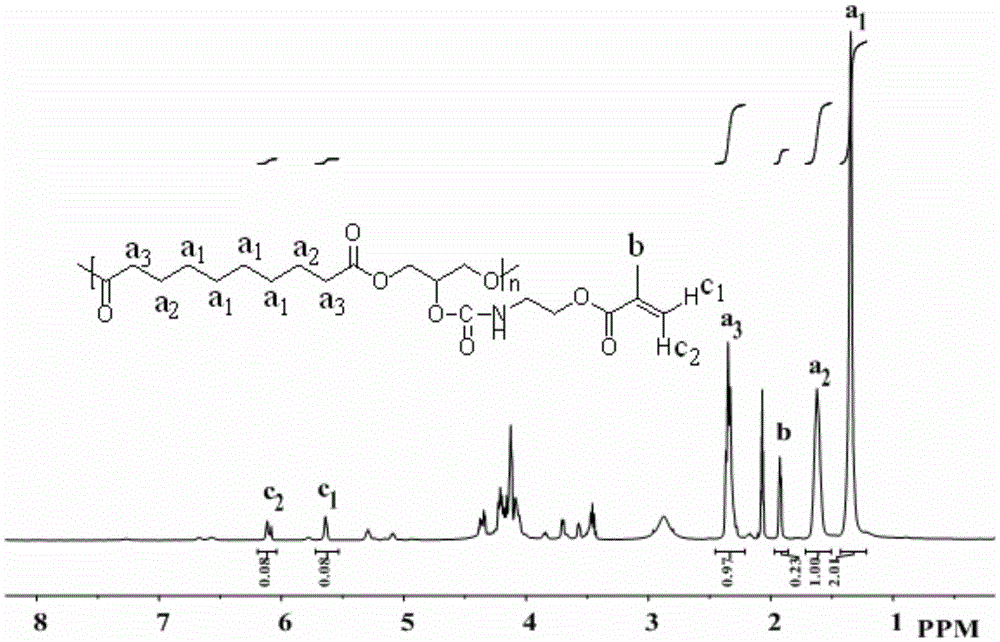
[0042]Preparation of PGS: Add 20.2574g (0.1mol) of sebacic acid (0.1mol) and 9.2037g (0.1mol) of glycerol after recrystallization and purification into a three-necked flask, melt at 135°C, and pass through N 2 Stir for 24h, then evacuate (4mbar) at 135°C for 48h, cool down to room temperature to give a pale yellow waxy solid.
[0043] 1 HNMR (400MHz, Chloroform-d) δ 3.45-5.29 (m, 5H), 2.35 (m, 4H), 1.62 (m, 4H), 1.35 (m, 4H).
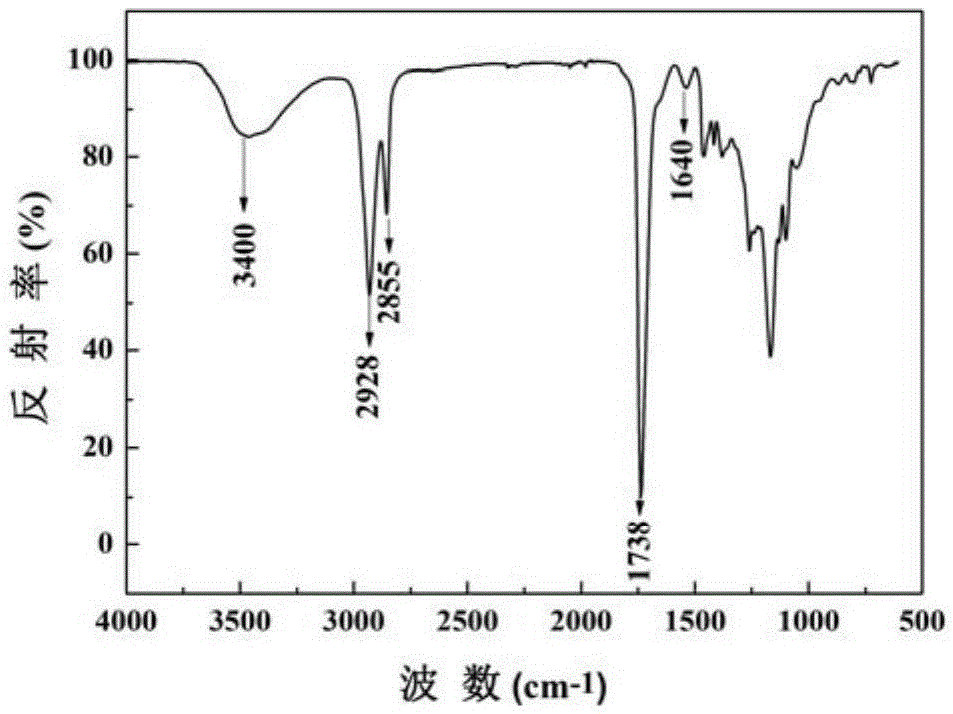
[0044] Synthesis of PGS-IM: The feeding of the reaction is carried out in a glove box (water content ≤ 0.1ppm, oxygen content ≤ 0.1ppm), weighing 1210.0mg (4.69mmol) PGS into a 25ml eggplant-shaped reaction bottle, and then adding 1.6ml without DMF in water, under nitrogen protection, placed in an 80°C oil bath and stirred until PGS was completely dissolved. 359.5 mg (2.35 mmol) of (2-isocyanoethyl)methacrylate were added and the reaction was complete after 20 minutes. Precipitate with deionized water to remove DMF, then dissolve with tetrahydrofuran...
Embodiment 2
[0053] Preparation of PGS: Add 20.2574g (0.1mol) of sebacic acid (0.1mol) and 9.2037g (0.1mol) of glycerol after recrystallization and purification into a three-necked flask, melt at 135°C, and pass through N 2 Stir for 24h, then evacuate (4mbar) at 135°C for 48h, cool down to room temperature to give a pale yellow waxy solid.
[0054] Synthesis of PGS-IM: The feeding of the reaction is carried out in a glove box (water content ≤ 0.1ppm, oxygen content ≤ 0.1ppm), weighing 4.69mmol) PGS is added to a 25ml eggplant type reaction bottle, and then 1.6ml anhydrous DMF is added, Under nitrogen protection, place in an 80°C oil bath and stir until the PGS is completely dissolved. Add 4 mmol (2-isocyanoethyl) methacrylate, and the reaction ends after 20 minutes. Precipitate with deionized water to remove DMF, then dissolve with tetrahydrofuran, and precipitate with deionized water three times to remove unreacted (2-isocyanoethyl) methacrylate monomer to obtain a white viscous semi-sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
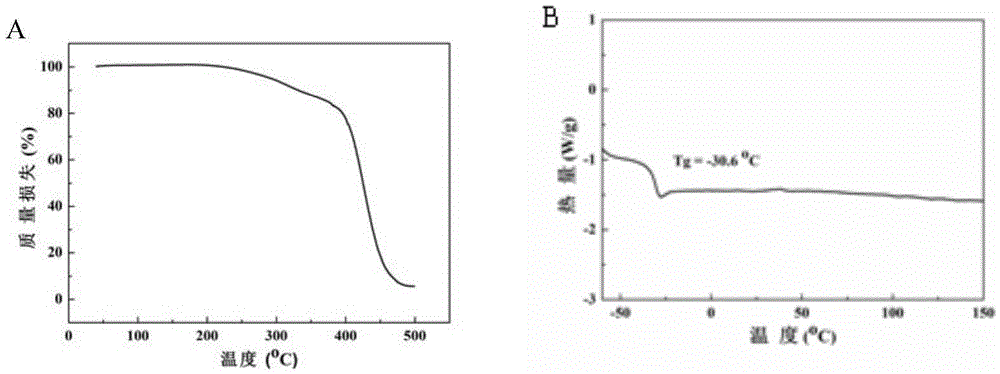
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com