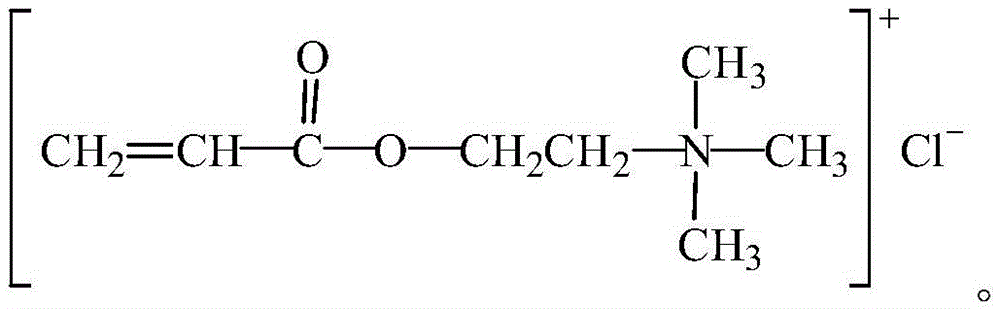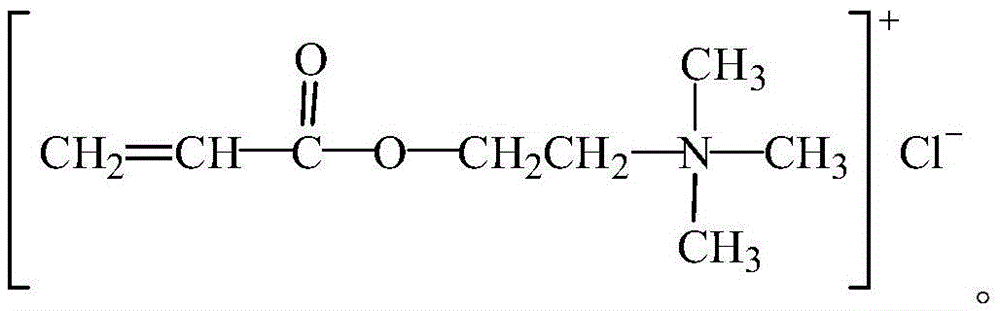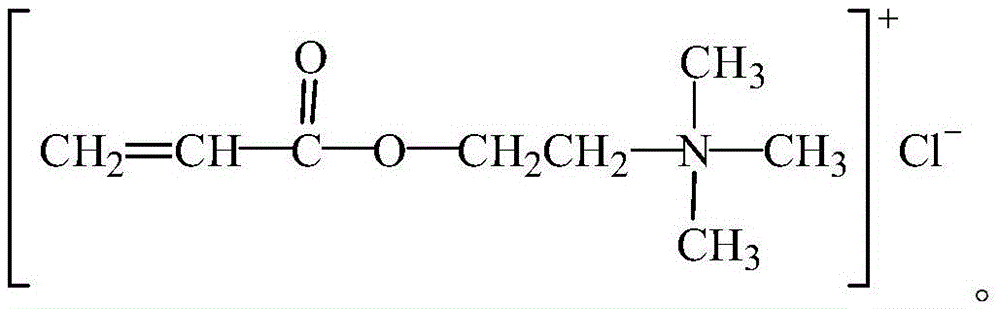Organic monomer for synthesizing amphoteric polycarboxylic superplasticizer and preparation method
A high-efficiency water reducer and polycarboxylic acid technology, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, organic chemistry, etc., can solve the problems of high synthesis cost and difficult industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of dimethylaminoethyl acrylate intermediate
[0023] Using one-time feeding method, add ethyl acrylate, N,N-dimethylaminoethanol, polymerization inhibitor and catalyst into a three-neck round bottom flask, turn on the stirring device, slowly heat up, condense and reflux for collection. During the preparation process, maintain the reaction temperature in the round bottom flask at 110°C to 130°C, control the temperature at the top of the fractionating column at 85°C to 95°C, and the reflux ratio to be 1:1 to 1:6, and the ethanol generated by the transesterification reaction and Ethyl acrylate azeotrope was distilled out of the system. After 4-8 hours of the transesterification reaction, stop the reaction to obtain the crude product of dimethylaminoethyl acrylate. The obtained crude product is subjected to sedimentation and vacuum distillation to obtain the dimethylaminoethyl acrylate intermediate for future use.
[0024] Preferably, the molar ratio of et...
Embodiment 2
[0035] It is basically the same as Example 1, the difference is:
[0036] Preferably, the molar ratio of ethyl acrylate to N,N-dimethylaminoethanol in the step (1) is 1:1.
[0037] Preferably, the polymerization inhibitor in the step (1) is a phenothiazine polymerization inhibitor, and the amount of the polymerization inhibitor is 0.2% of the total mass of ethyl acrylate and N,N-dimethylaminoethanol.
[0038] Preferably, the catalyst in the step (1) is a sodium methoxide catalyst, and the catalyst dosage is 1.0% of the total mass of ethyl acrylate and N,N-dimethylaminoethanol.
[0039] Preferably, the molar ratio of water to dimethylaminoethyl acrylate intermediate in the step (2) is 1.5:1.
[0040] Preferably, the polymerization inhibitor in the step (2) is a phenothiazine polymerization inhibitor, and the amount of the polymerization inhibitor is 0.25% of the mass of the dimethylaminoethyl acrylate intermediate.
Embodiment 3
[0042] Basically the same as Example 1, the difference is:
[0043] Preferably, the molar ratio of ethyl acrylate to N,N-dimethylaminoethanol in the step (1) is 1:1.
[0044] Preferably, the polymerization inhibitor in the step (1) is a 2,2,6,6-tetramethylpiperidine oxide polymerization inhibitor, and the amount of the polymerization inhibitor is ethyl acrylate and N,N-dimethylamino 0.3% of the total mass of ethanol.
[0045] Preferably, the catalyst in the step (1) is a tetrabutyl titanate catalyst, and the amount of the catalyst is 1.5% of the total mass of ethyl acrylate and N,N-dimethylaminoethanol.
[0046] Preferably, the molar ratio of water to dimethylaminoethyl acrylate intermediate in the step (2) is 2.0:1.
[0047] Preferably, the polymerization inhibitor in the step (2) is a hydroquinone polymerization inhibitor, and the polymerization inhibitor consumption is 0.5% of the mass of the dimethylaminoethyl acrylate intermediate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com