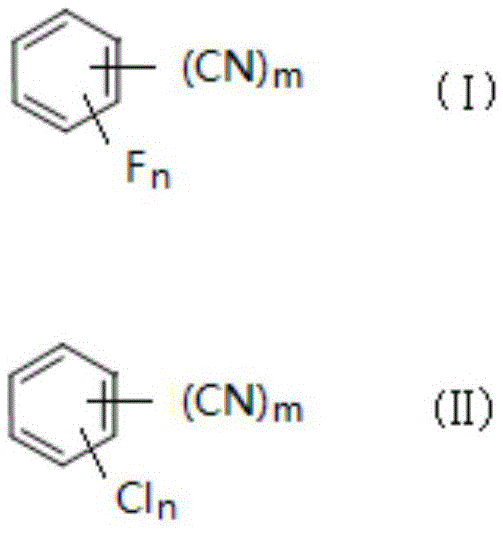
Fluorobenzonitrile compound preparation method
A technology of fluorobenzonitriles and compounds, which is applied in the field of preparation of fluorobenzonitriles, to achieve the effects of saving resources, speeding up the reaction, and solving the complex separation process of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 5
[0079] The preparation of embodiment 1 pentafluorobenzonitrile
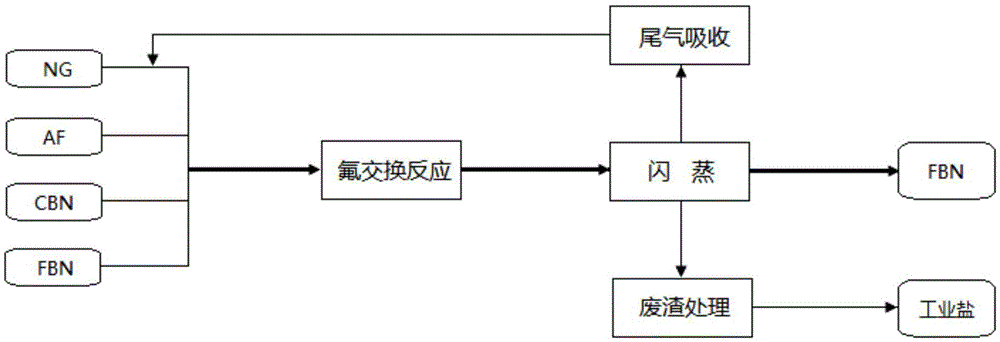
[0080] ① Fluorination: In a 10L fluorination autoclave equipped with a flash condenser and a receiving tank, add 1000g (3.6mol) of pentachlorobenzonitrile, 1280g (21.6mol) of anhydrous potassium fluoride and pentafluorobenzonitrile respectively. Nitrile 3000g (15.4mol), nitrogen replacement three times, remove the air in the kettle, pressurize the nitrogen to 1MPa, airtight, raise the temperature of the kettle to 280-300°C in the stirring state, keep it warm for 20h, the maximum gauge pressure of the reactor can reach 2.1MPa, Gas chromatographic analysis was performed on the still liquid sample, and the content of pentafluorobenzonitrile was 95.6%.
[0081] ②Flash: first pre-cool the flash condenser and tail gas trap on the receiving tank with the cooling medium, then slowly open the flash outlet valve of the fluorinated autoclave, and control the temperature of the condensate in the flash condenser not to exceed...
Embodiment 2 5
[0082] The preparation of embodiment 2 pentafluorobenzonitriles
[0083] ① Fluorination: In a 10L fluorination autoclave equipped with a flash condenser and a receiving tank, add 1000g (3.6mol) of pentachlorobenzonitrile, 1280g (21.6mol) of anhydrous potassium fluoride and pentafluorobenzonitrile respectively. Nitrile 5000g (25.6mol), nitrogen replacement three times, remove the air in the kettle, pressurize the nitrogen to 1MPa, airtight, raise the temperature of the kettle to 320-340°C in the stirring state, keep it warm for 20h, the maximum gauge pressure of the reactor can reach 2.8MPa, Gas chromatographic analysis was performed on the still liquid sample, and the content of pentafluorobenzonitrile was 98.7%.
[0084] ②Flash: first pre-cool the flash condenser and tail gas trap on the receiving tank with the cooling medium, then slowly open the flash outlet valve of the fluorinated autoclave, and control the temperature of the condensate in the flash condenser not to excee...
Embodiment 3
[0085] The preparation of embodiment 3 pentafluorobenzonitriles
[0086] ① Fluorination: In a 10L fluorination autoclave equipped with a flash condenser and a receiving tank, add 1000g (3.6mol) of pentachlorobenzonitrile, 1280g (21.6mol) of anhydrous potassium fluoride and pentafluorobenzonitrile respectively. Nitrile 4000g (20.5mol), nitrogen replacement three times, remove the air in the kettle, pressurize the nitrogen to 1MPa, seal it, raise the temperature of the kettle to 240-250°C in the stirring state, keep it warm for 8h, the maximum gauge pressure of the reactor can reach 1.6MPa, Gas chromatographic analysis was performed on the still liquid sample, and the content of pentafluorobenzonitrile was 95.1%.
[0087] ②Flash: first pre-cool the flash condenser and tail gas trap on the receiving tank with the cooling medium, then slowly open the flash outlet valve of the fluorinated autoclave, and control the temperature of the condensate in the flash condenser not to exceed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com