Application of kelimycin in mycobacterium tuberculosis infection resistance
A technology of mycobacteria and carrimycin, applied in medical preparations containing active ingredients, antibacterial drugs, organic active ingredients, etc., can solve problems such as limited bactericidal effect, low cure rate, and poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] "Example 1" acquisition and processing of Mycobacterium tuberculosis specimens
[0015] According to the provisions of the national standard WS288-2008 "Diagnostic Criteria for Pulmonary Tuberculosis" issued by the Ministry of Health, select patients with confirmed or highly suspected tuberculosis with clinical symptoms, signs, and chest imaging examinations. Collect about 2 mL of sputum, pleural effusion, cerebrospinal fluid, and pus samples from the selected patients, and add them to a 50 mL centrifuge tube with a screw cap. Add N-acetyl-L-cysteine sodium hydroxide (NaOH-NALC) pretreatment solution equal to that of the sample, and vortex for 20 seconds. Let stand at room temperature for 18 minutes. Add PBS (pH6.8) to 40mL, centrifuge at 3000g for 20 minutes, then discard the supernatant and keep the precipitate. 2 mL of LPBS (pH 6.8) was added to prepare a suspension. The treated specimens were inoculated into medium for solid culture.
Embodiment 2
[0016] "Example 2" Isolation and Culture Identification of Mycobacterium Tuberculosis Specimen
[0017] 1. Preparation of culture medium:
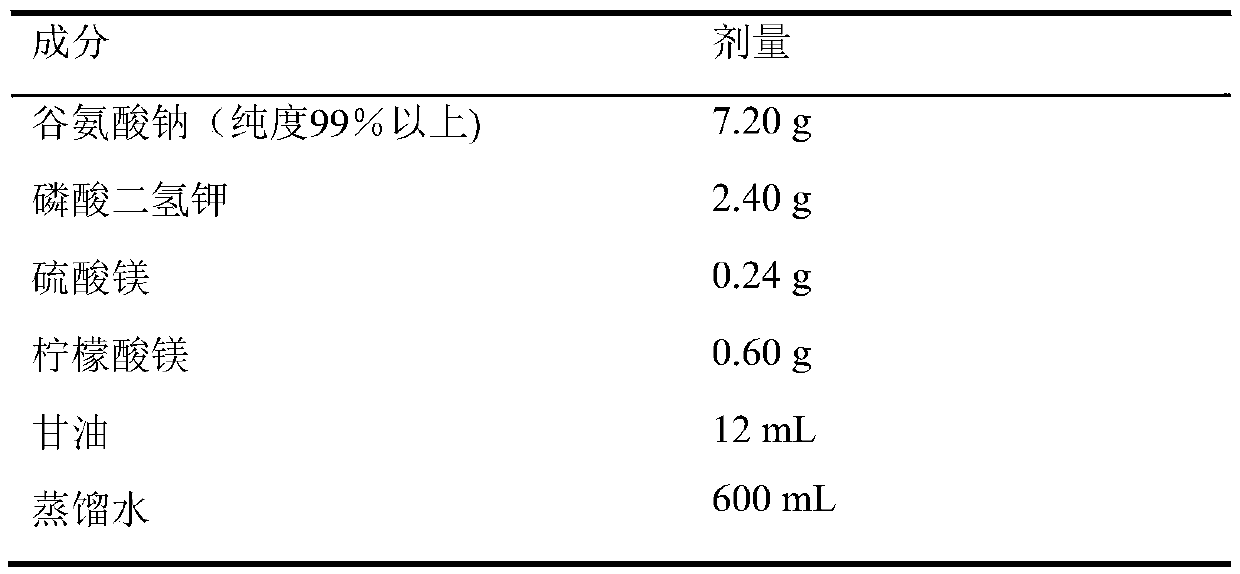
[0018] The composition of the medium is shown in Table 1. Add each ingredient into distilled water according to the listed dosage, fully dissolve; boil for 30 minutes or 121 ℃ high pressure for 15 minutes.
[0019] Take fresh chicken eggs, wash them with tap water, scrub them with soapy water, and wipe them with 75% alcohol for disinfection after drying. Under aseptic operation, pour the egg liquid into a sterilized graduated enamel cup, stir and mix well, after filtering with sterilized gauze, take 1000mL and add it, mix well; add 20mL of 2% malachite green, mix well; Add 7mL of culture medium into a test tube (18mm×180mm), and put it in a steam incubator at 85°C for 50 minutes to solidify. Take the prepared culture medium in the tube at 5%, put it into 37°C for 48 hours, and carry out the sterility test; after passing the sterility te...
Embodiment 3
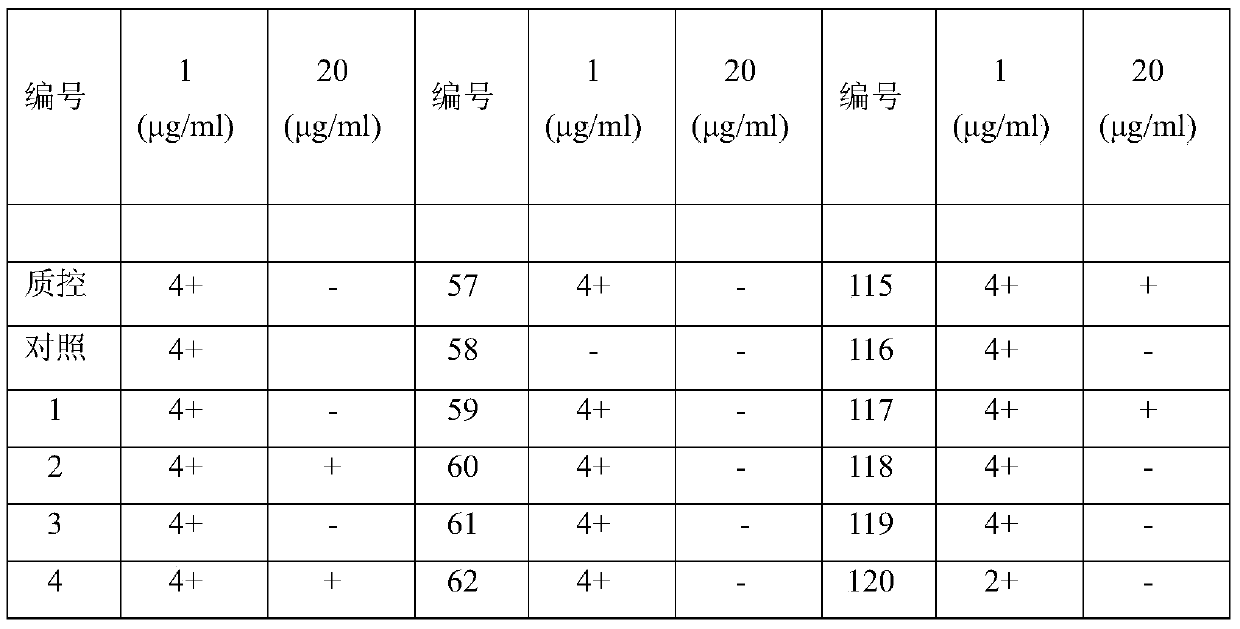
[0026] "Example 3" Carrimycin anti-mycobacterium tuberculosis activity test
[0027] 1. Absolute concentration method
[0028] 1) Anti-TB drugs
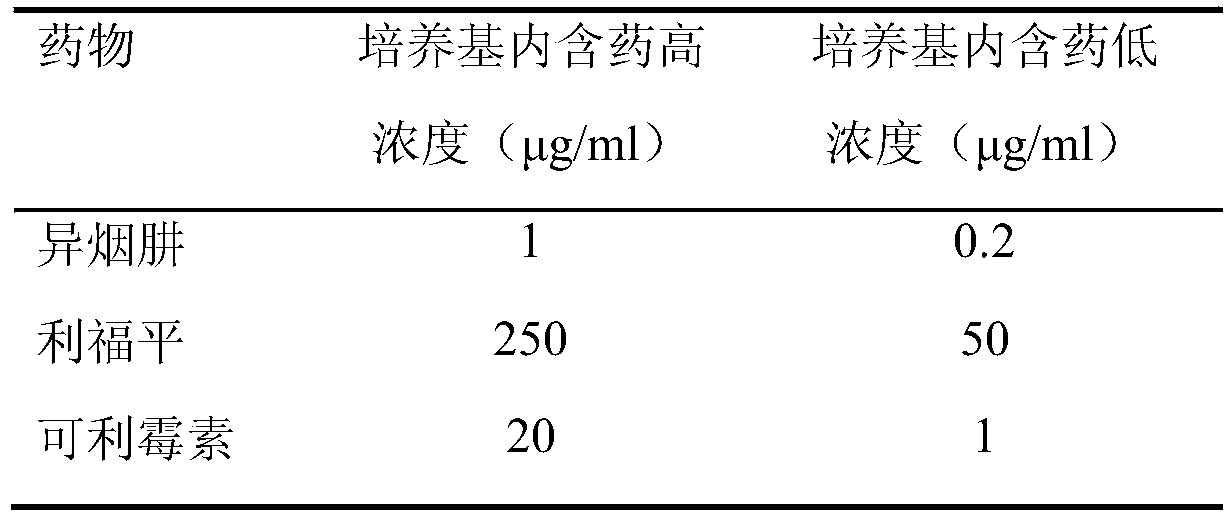
[0029] Carrimycin standard product: from China National Institute for the Control of Pharmaceutical and Biological Products; reference drugs: isoniazid and rifampicin are standard products from Sigma Company. Anti-tuberculosis drugs were formulated into mother solutions at a certain concentration, and then added to the culture medium in a certain amount to prepare the required dose (Table 2).
[0030] 2) Strain inoculation
[0031] The strains isolated from clinical specimens were confirmed as cultures of acid-fast bacteria by smear, diluted with normal saline containing 10% Tween 80, and compared with McFarland standard turbidimetric tubes (Guangdong Huankai Microbiology Technology Co., Ltd.), and prepared 10 -2 mg / mL bacterial liquid, inoculated in the medium containing the test drug.
[0032] Negative and positive controls wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com