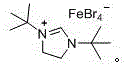Method for preparing carboxylate compounds
A technology of ester compounds and carboxylic acid compounds, which is applied in the field of preparation of organic compounds, can solve the problems of no esterification reaction between carboxylic acid and toluene compounds, no catalytic activity, and poor activity of allyl hydrocarbon compounds. The synthesis is simple, the catalytic efficiency is improved, and the effect of a single component
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment one [( t BuNCH 2 CH 2 N t Bu)CH][FeBr 4 ] Catalyzed esterification of benzoic acid with toluene
[0022] Add benzoic acid (61.1 mg, 0.5 mmol), catalyst (14.0 mg, 0.025 mmol, 5mol%), di-tert-butyl peroxide (232 μl, 1.25 mmol), toluene (3 ml ), reacted at 110°C for 24 hours, cooled to room temperature after the reaction, and purified the product by column chromatography (using a mixed solvent with a volume ratio of ethyl acetate / petroleum ether of 1:100 as the developing solvent), and the yield was 94%. .
[0023] Dissolve the product in CDCl 3 Medium (about 0.4mL), seal the tube, measure and characterize on a UnityInova-400 NMR instrument at room temperature: 1 HNMR (400MHz, CDCl 3 , TMS): 8.07-8.09 (m, 2H), 7.52-7.56 (m, 1H), 7.32-7.46 (m, 7H), 5.36 (s, 2H).
Embodiment 2
[0024] Embodiment two [( t BuNCH 2 CH 2 N t Bu)CH][FeBr 4 ] Catalyzed esterification of p-methylbenzoic acid with toluene
[0025] Add p-toluic acid (68.1 mg, 0.5 mmol), catalyst (28.0 mg, 0.05 mmol, 10 mol%), di-tert-butyl peroxide (232 μl, 1.25 mmol) and toluene in sequence in the reaction flask (3 ml), reacted at 100°C for 28 hours, cooled to room temperature after the reaction, and the product was purified by column chromatography (petroleum ether was used as developing solvent), and the yield was 96%.
[0026] Dissolve the product in CDCl 3 Medium (about 0.4mL), seal the tube, measure and characterize on a UnityInova-400 NMR instrument at room temperature: 1 HNMR (400MHz, CDCl 3 , TMS): 8.00 (d, 2H), 7.46-7.48 (m, 2H), 7.35-7.42 (m, 3H), 7.25 (d, 2H), 5.37 (s, 2H), 2.41 (s, 3H).
Embodiment 3
[0027] Embodiment three [( t BuNCH 2 CH 2 N t Bu)CH][FeBr 4 ] Catalyzed esterification of m-trimethylbenzoic acid with toluene
[0028]Add m-trimethylbenzoic acid (82.1 mg, 0.5 mmol), catalyst (14.0 mg, 0.025 mmol, 5mol%), di-tert-butyl peroxide (187 microliters, 1.00 mmol) successively in the reaction flask, Toluene (3 milliliters) was reacted at 110°C for 24 hours, cooled to room temperature after the reaction, and the product was purified by column chromatography (using ethyl acetate / petroleum ether volume ratio of 1:50 mixed solvent as developing solvent) to produce The rate is 91%.
[0029] Dissolve the product in CDCl 3 Medium (about 0.4mL), seal the tube, measure and characterize on a UnityInova-400 NMR instrument at room temperature: 1 HNMR (400MHz, CDCl 3 , TMS): 7.40-7.43 (m, 2H), 7.28-7.36 (m, 3H), 6.81 (s, 2H), 5.33 (s, 2H), 2.24 (s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com