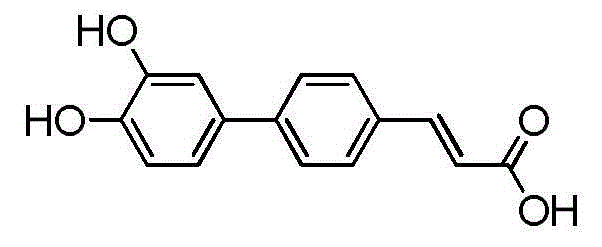
Application of biphenyl compound 4-(3', 4'-dihydroxy phenyl) cinnamic acid in drug preparation
A technology of dihydroxyphenyl and compounds, applied in the field of medicine, can solve problems that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0017] Example preparation of 14-(3',4'-dihydroxyphenyl) cinnamic acid
[0018] Take 25kg of dry roots of Sesame chinensis, crush them, and reflux extract with 95% ethanol for 3 times (50L×3), each time for 2h, combine the extracts and concentrate to obtain 0.95kg of extract, add water (4L) to suspend, and add an equal volume of petroleum Extracted 5 times with ether, ethyl acetate and n-butanol, combined the extracts and concentrated to dryness to obtain 200 g of ethyl acetate extract. The ethyl acetate extraction part was separated by silica gel (200-300 mesh) column chromatography, and sequentially eluted with dichloromethane-methanol (50:1-0:1) gradient to obtain 11 fractions (Fr.1-11) , wherein the fraction Fr.3 (32g) was subjected to silica gel column chromatography (dichloromethane-methanol, 30:1, 20:1, 10:1, 5:1, 3:1, 1:1), MPLC (methanol -water, 20:80-80:20 gradient elution) and semi-preparative HPLC and other means to purify, isolate and obtain compound 4-(3',4'-dih...
Embodiment 2
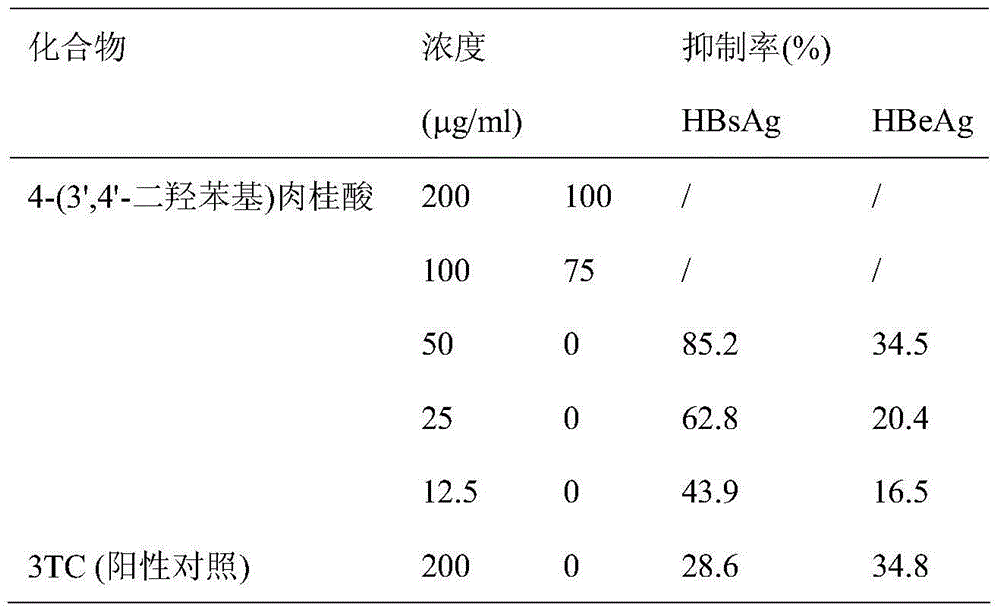
[0020] Embodiment 2 in vitro anti-HBV experiment
[0021] 2.2.15 cell line of HepG2 (Ministry of Education / Ministry of Health Key Laboratory of Medical Molecular Virology, Shanghai), with 10×10 cells per well 5 Cells were inoculated in a 24-well plate, the medium was DMEM, the growth medium contained 10% fetal bovine serum, 380 μg / ml G418, 0.03% glutamine, 100 μg / ml each of penicillin and streptomycin, in 5% CO 2 Cultivate in an incubator at 37°C. After 48 hours, replace it with a drug-containing culture solution aided by dimethyl sulfoxide. Set 3 to 5 concentrations for each drug, and set 4 parallel wells for each concentration, and continue to cultivate for 9 days. (Change the medium once every 3 days), collect the supernatant and detect the content of HBsAg and HBeAg by ELISA. Under the same conditions, the culture supernatant without drugs was used as the control group. At the same time, the above cell lines were used to measure the cytotoxicity of the drug by MTT method...
Embodiment 3
[0025] Example 3 Anti-complement classical pathway activation test in vitro
[0026] Take 0.04ml of complement (guinea pig serum), add barbiturate buffer solution (BBS) to prepare a 1:10 solution, and double-dilute with BBS to 1:20, 1:40, 1:80, 1:160, 1:10 320, 1:640 and 1:1280 solutions. Take 1:1000 hemolysin, 0.1ml of 2% sheep red blood cells (SRBC) and 0.2ml of complement of each concentration, dissolve them in 0.2ml of BBS, mix well, put them in a low-temperature high-speed centrifuge at 4000rpm and 4℃ Centrifuge for 5min. Take 0.2ml of the supernatant from each tube and place it in a 96-well plate, and measure its absorbance at 405nm. At the same time, a complete hemolysis group (0.1ml 2% SRBC, 0.1ml hemolysin dissolved in 0.4ml triple distilled water) was set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Plot the dilution of complement on the X-axis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com