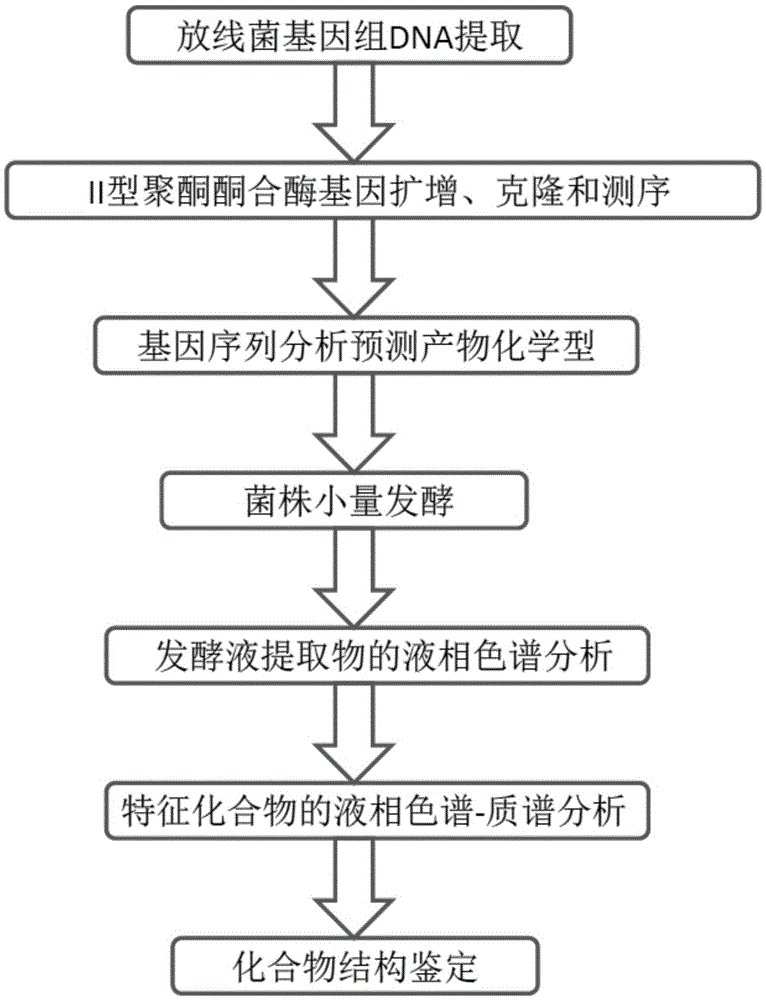
Aromatic ring polyketide finding and identifying method mediated through gene analysis
A gene analysis and identification method technology, which is applied in the field of rapid search and identification of actinomycete-derived aromatic ring polyketide compounds mediated by gene analysis, can solve the problem that there is no gene analysis to guide the directional discovery of compounds, and achieve the goal of improving the discovery efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
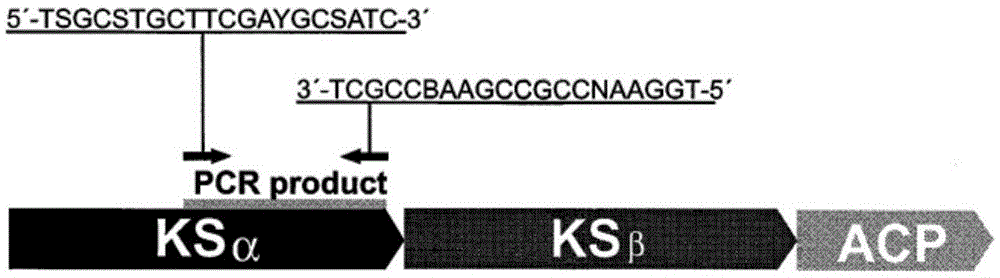
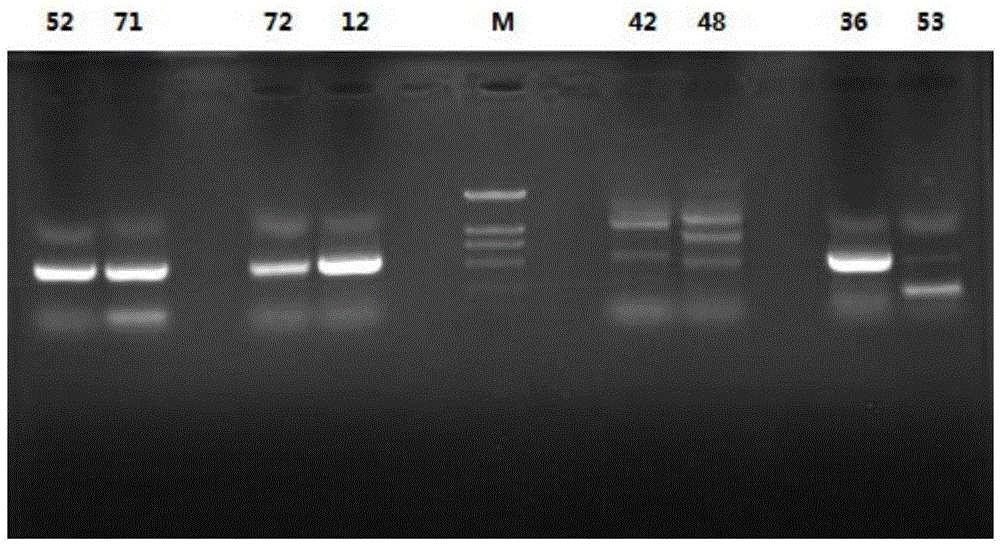
[0034] Example 1. Through the amplification and screening of the type II polyketide synthase gene of the strain to be tested, it has aromatic ring polyketide synthesis potential force strain
[0035] This example provides the specific operation and screening results of screening aromatic ring polyketide synthesis potential strains from actinomycete strains derived from sponges:
[0036] 1. Extraction of the genomic DNA of the strain to be tested
[0037] The extraction method is as follows: collect the bacteria, add 450 μl TE buffer, vortex and mix; add 50 μl 1% lysozyme, mix well, and bathe in water at 37°C for 30 minutes; add 50 μl 0.5 MEDTA, 50 μl 10% SDS, 10 μl 0.1% proteinase K, 55 ℃ water bath for 60min; add 1 / 2 volume of 7.5M ammonium acetate, mix well; centrifuge at 12000rpm for 20min, transfer the supernatant to a new tube; add an equal volume of isopropanol, precipitate at -20℃ for 30min, centrifuge at 12000rpm for 10min to collect the precipitate; add 70% The pre...
Embodiment 2
[0050] Example 2, by comparing the amino acid sequences of the reading frames of the amplification products and analyzing the phylogenetic evolution to predict the product Physicochemical type
[0051] This example provides a phylogenetic analysis of the amino acid sequences of 18 ketone synthase genes of the 17 aromatic ring polyketide synthesis potential strains described in Example 1.
[0052] The result is as Figure 4 shown. The amino acid sequences of the ketosynthase genes of 3 actinomycete strains S.rocheiS41, S.anulatusS71, and N.araoensisS107 are clustered in the polyketide cluster of anthracyclines, suggesting that these three strains of actinomycetes have the ability to synthesize anthracyclines Ability.
Embodiment 3
[0053] Embodiment 3, the step of fermenting described bacterial strain and searching for, identifying described chemotype product in fermented liquid extract
[0054] This example provides the results of the small-scale fermentation of the three anthracycline polyketide-synthesizing potential strains described in Example 2 and the results of the search and identification of the target compound.
[0055] 1. A small amount of shake flask fermentation of the actinomycetes strain and the organic solvent extraction of the fermented liquid
[0056] The fermentation conditions are as follows: add 100ml GYM4 medium (10g glucose, 4g yeast extract powder, 4g malt extract powder, 1L distilled water, pH7.2) to a 250ml Erlenmeyer flask, place it in a shaker after inoculation, and place it at 28°C at a speed of 120rpm Cultured for 5 days.
[0057] The extraction method is specifically as follows: the fermented liquid is filtered by suction to remove bacteria, the filtrate is added with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com