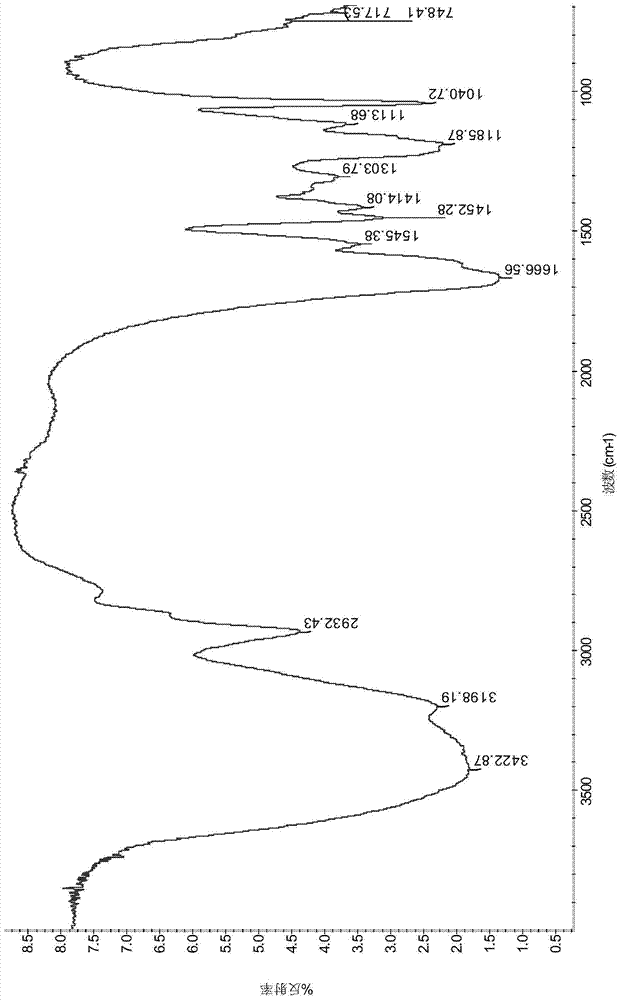
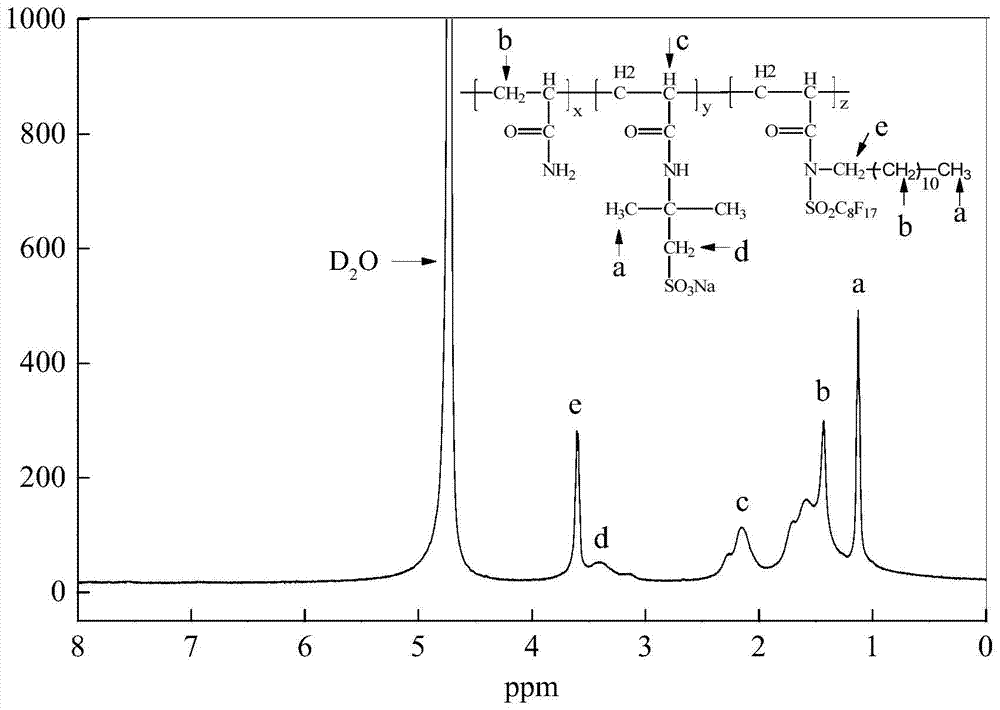
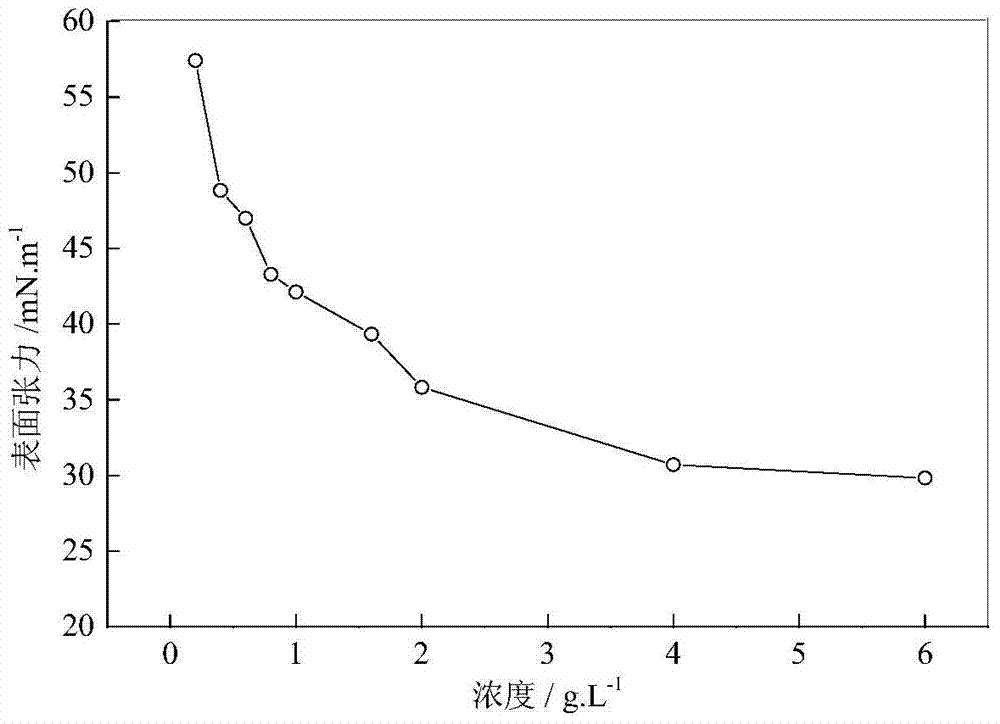
A kind of fluorine-containing double tail hydrophobic association polymer and its preparation method
A hydrophobic association and polymer technology, applied in the direction of drilling compositions, chemical instruments and methods, etc., can solve the problems of poor oil displacement effect and weakened synergistic effect of oil displacement agents, and achieve low surface tension and strong enhancement viscous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] Preferably, the preparation method of the fluorine-containing double tail hydrophobic association polymer comprises the following steps:
[0023] Step S1, after mixing 1-2 mole fractions of long-chain alkylamine with 1-2 mole fractions of triethylamine and an organic solvent, add 1 mole fraction of perfluorooctanesulfonyl Fluorine, then raise the temperature to 30-60°C, continue the reaction for 4-10h, the reaction mixture is washed, dried, and distilled under reduced pressure to obtain the product N-alkyl perfluorooctane sulfonamide.
[0024] Further preferably, the organic solvent in step S1 is one or more of toluene, isopropanol, isopropyl ether, acetone or xylene.
[0025] Further preferably, the long-chain alkylamine in step S1 is one of n-hexylamine, n-octylamine, dodecylamine, tetradecylamine, cetylamine, and octadecylamine.
[0026] Further preferably, the reaction mixture in step S1 is washed successively with water, 0.5% hydrochloric acid aqueous solution, sa...
Embodiment 1
[0035] The first step: Synthesis of fluorine-containing double tail polymerizable macromonomer AMPD
[0036] S1, Synthesis of N-dodecyl perfluorooctane sulfonamide
[0037] Add 0.15 mol of dodecylamine, 0.2 mol of triethylamine and 100 mL of isopropyl ether into a 250 mL three-necked flask, mix well and slowly add 0.12 mol of perfluorooctanesulfonyl fluoride dropwise under stirring conditions. Then the temperature was raised to 50°C, and the reaction was continued for 5h. The reaction mixture was washed successively with water, 0.5% hydrochloric acid aqueous solution, and saturated brine, then dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain the product N-alkylperfluorooctanesulfonamide.
[0038] S2, Synthesis of Macromonomer AMPD
[0039] Add 0.1 mol of N-dodecyl perfluorooctane sulfonamide to the three-necked flask, and add dichloromethane to form a 40% solution. Stir vigorously and blow nitrogen gas, control the temperature at 3°C, s...
Embodiment 2
[0048] The first step: Synthesis of fluorine-containing double tail polymerizable macromonomer AMPD
[0049] S1, Synthesis of N-dodecyl perfluorooctane sulfonamide
[0050] Add 0.15 mol of dodecylamine, 0.18 mol of triethylamine and 120 mL of acetone into a 250 mL three-necked flask, mix well and slowly add 0.12 mol of perfluorooctanesulfonyl fluoride dropwise under stirring conditions. Then the temperature was raised to 45°C, and the reaction was continued for 5h. The reaction mixture was washed successively with water, 0.5% hydrochloric acid aqueous solution, and saturated brine, then dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain the product N-dodecylperfluorooctanesulfonamide.
[0051] S2, Synthesis of Macromonomer AMPD
[0052] Add 0.15 mol of N-dodecyl perfluorooctane sulfonamide to the three-necked flask, and add dichloromethane to form a 40% solution. Stir vigorously and blow nitrogen gas, control the temperature at 4°C, slowl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com