Application of moroxydine in preparing myocardial infarction therapeutic drug
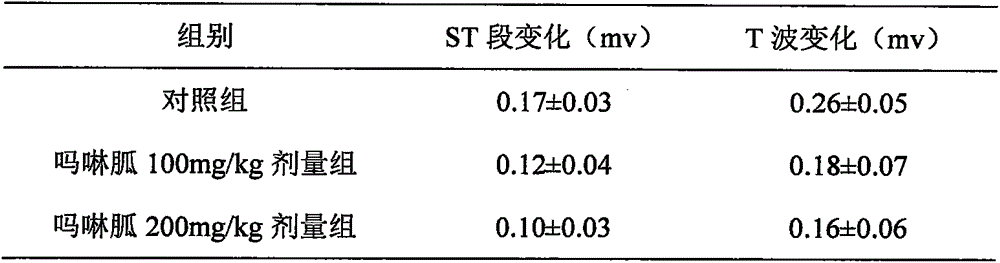
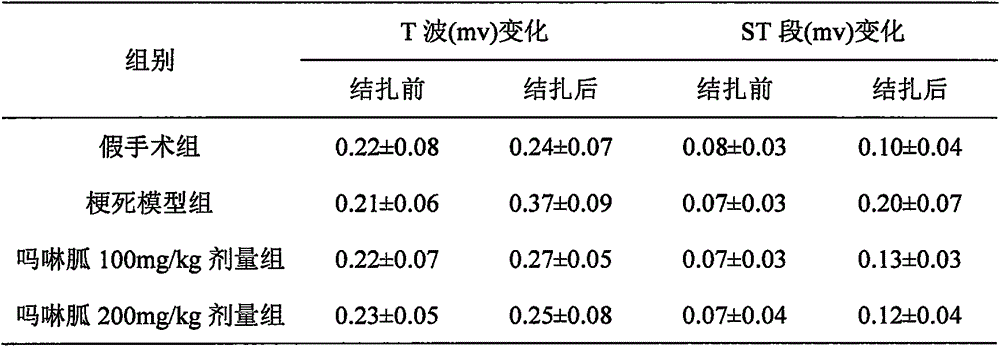
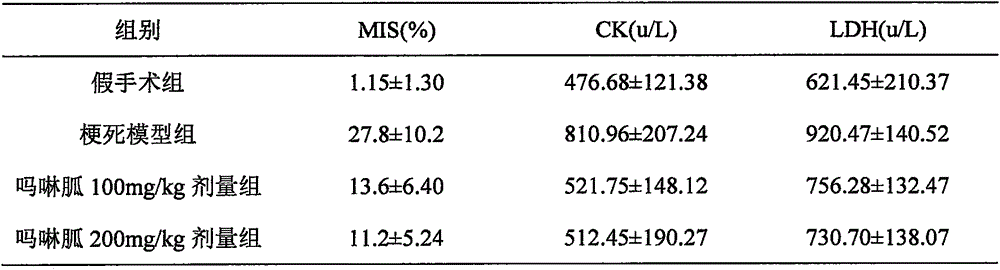
A technique for myocardial infarction and a drug for treating myocardial infarction is applied to the new medical use of morpholine guanidine. The application field of morpholine guanidine for preparing a drug for treating myocardial infarction can solve the problems of poor efficacy of the drug for treating myocardial infarction and the like, and achieve a reduction in serum muscle mass. Acid kinase activity, regulation of vascular tone, significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] Specific embodiments of the present invention will be described in detail below. In order to avoid too many unnecessary details, well-known structures or functions will not be described in detail in the following embodiments.
[0016] Approximate language used in the following examples is for quantitative representations, indicating that certain variations in quantities are permissible without altering essential function. Accordingly, values modified by language such as "about", "approximately" and the like are not limited to the exact value itself. In some embodiments, "about" means that the corrected value is allowed to vary within the range of plus or minus ten percent (10%), for example, "about 100" means any value between 90 and 110. Furthermore, in the expression "about the first value to the second value", both values of the first value and the second value are corrected approximately at the same time. In some cases, the language of approximation may relate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com