Lobaplatin crystal and preparation method and drug application thereof
A technology of lobaplatin trihydrate and lobaplatin, which is applied to a new crystal form and a preparation method thereof and the application field in medicine, can solve the problems of difficulty in preparing preparations, poor stability and the like, and achieves good fluidity and good stability. , the effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] On the other hand, the present invention provides a method for preparing a new crystal form of lobaplatin that is simple to prepare, easy to operate, and suitable for scale-up production.
[0054] In a preferred embodiment, the preparation method of the new crystal form of lobaplatin of the present invention comprises the following steps:
[0055] a. Preparation of lobaplatin dihydrate: Weigh lobaplatin trihydrate in a container, add 15-30ml organic solvent to 1g lobaplatin trihydrate, suspend and stir at room temperature for 45-50h, filter and wash with ether , to obtain a white powder after vacuum drying, which is lobaplatin dihydrate;
[0056] Wherein, the organic solvent is selected from methyl tert-butyl ether, toluene, diethyl ether, butyl acetate, 1,4-dioxane or n-heptane.
[0057] b. Preparation of the target crystal form: Weigh the lobaplatin dihydrate obtained in step a, put it in a container, add anhydrous methanol, stir at room temperature until the solid d...
Embodiment 1
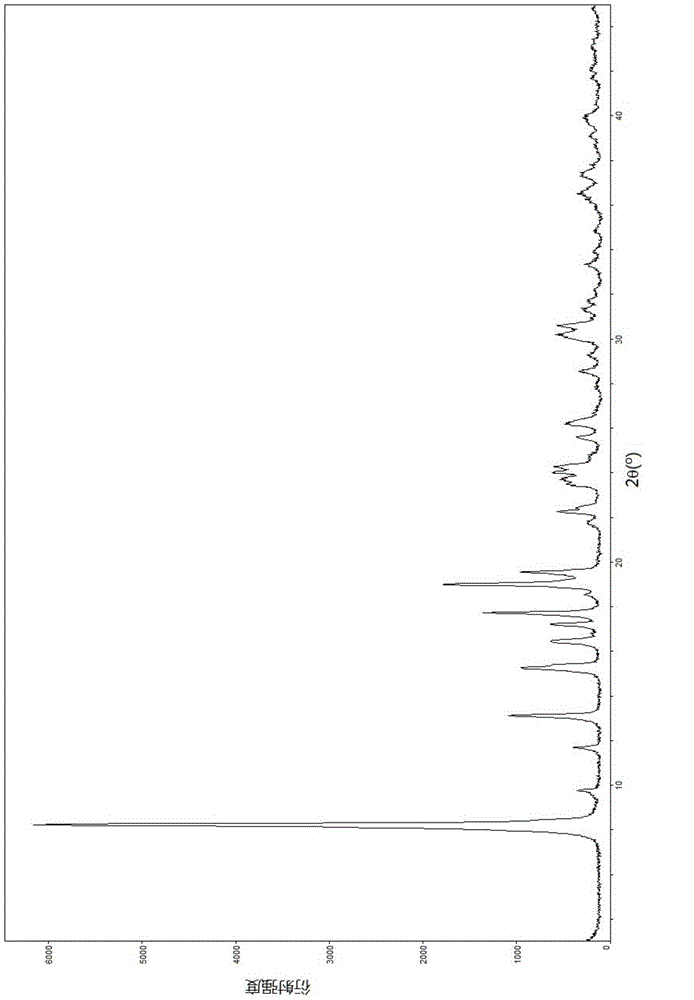
[0067] Embodiment 1: Screening analysis of each crystal form
[0068] 1.1 Screening by room temperature volatile crystallization method
[0069] Take 20 mg of lobaplatin trihydrate sample and put it into a 10 ml sample bottle, add 3 ml of absolute ethanol or absolute methanol, after fully dissolving, place it in an environment of 25°C and slowly volatilize to obtain a dry solid, which is then determined by PXRD. The results are shown in Table 2 below:
[0070] Table 2 normal temperature volatilization and crystallization test results
[0071] Numbering
solvent
PXRD (possible crystal number)
1-1
Absolute ethanol
B
1-2
B
[0072] The results showed that the crystal forms obtained in absolute methanol and absolute ethanol were compared and found to be the same crystal form, tentatively named as crystal form B.
[0073] 1.2 Screening by suspension crystallization method
[0074] Take 20mg of lobaplatin t...
Embodiment 2
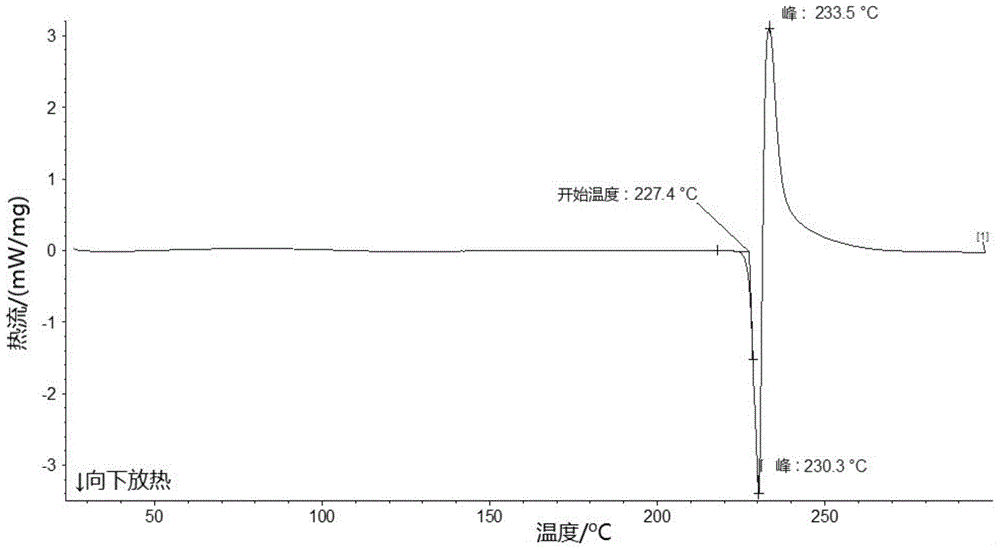
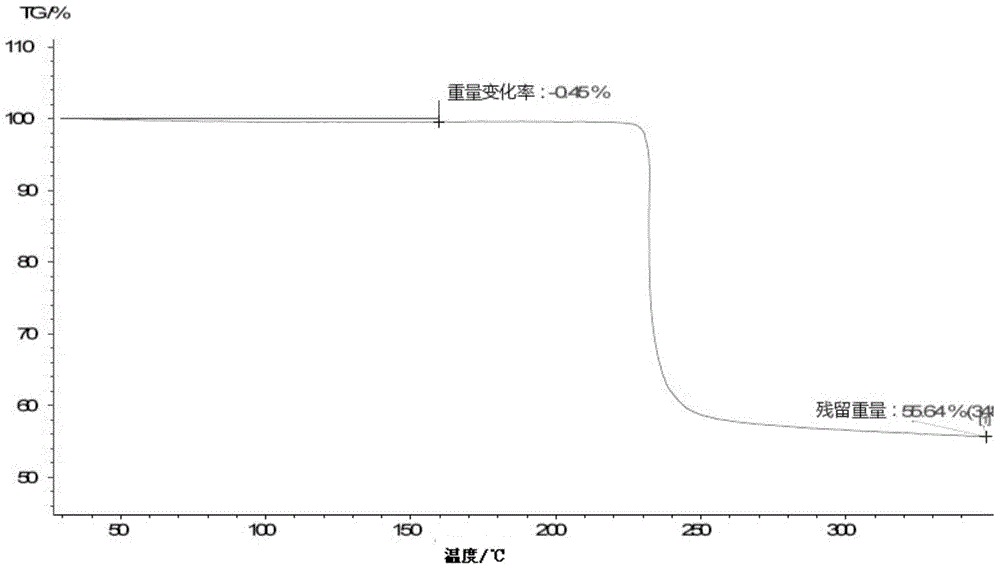
[0116] Example 2: Preparation of a new crystal form of lobaplatin named Form B
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com