High-selectivity hydrazine ratiometric fluorescent probe and preparation method thereof
A technology of molar ratio and compound, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effect of simple synthesis and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] (Scheme 1) 4-hydroxy-1,8-naphthalimide (269mg, 1.0mmol), 4-bromobutyric acid (166mg, 1.0mmol), 4-dimethylaminopyridine (122mg, 1.0mmol) and di Cyclohexylcarbodiimide (412mg, 2.0mmol) was dissolved in 20mL of dry dichloromethane, reacted at room temperature for 6h, evaporated under reduced pressure to obtain a crude product, and then separated by column chromatography using dichloromethane to obtain 179mg of a light yellow pure product , and the yield was 43%.
[0027] (Scheme 2) 4-hydroxy-1,8-naphthalimide (269mg, 1.0mmol), 4-bromobutyric acid (332mg, 2.0mmol), 4-dimethylaminopyridine (244mg, 2.0mmol) and di Cyclohexylcarbodiimide (618 mg, 3.0 mmol) was dissolved in 20 mL of dry dichloromethane, reacted at room temperature for 6 hours, evaporated under reduced pressure to obtain a crude product, and then separated by column chromatography using dichloromethane to obtain 283 mg of a light yellow pure product , and the yield was 68%.
[0028] (Scheme 3) 4-hy...
Embodiment 2
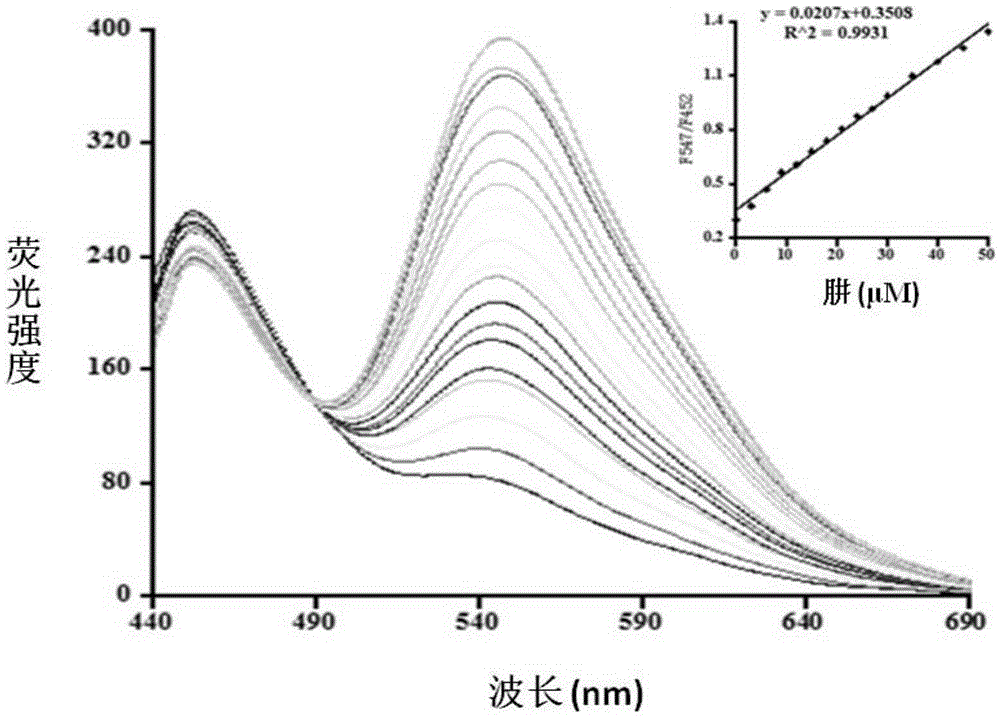
[0032] The inventor of the present invention has carried out following test: (a) the impact of different concentrations of hydrazine (0~100 μ M) on the probe (5 μ M) fluorescence spectrum; Insert figure is the ratio of fluorescence intensity at 547nm place and fluorescence intensity at 452nm place and added hydrazine The linear relationship between concentrations. The above determinations were made in a system containing ethanol and water (3:7, V / V) containing 5mM phosphate buffered saline (PBS), pH 7.4, and all spectral tests were measured at 25°C after adding hydrazine for 15 minutes of. See results figure 1 .
[0033] from figure 1 It can be seen that with the increase of the concentration of hydrazine in the probe solution, the fluorescence emission peak at 452nm decreases gradually, and at the same time, a new emission peak at 547nm occurs and gradually increases. The ratio of the intensity to the fluorescence intensity at 452nm has a good linear relationship. Theref...
Embodiment 3
[0035] Effect of different analytes (50 μM) on the fluorescence spectra of probes (5 μM). Analytes include: ethylenediamine EDA, dimethylamine DMA, triethylamine TEA, n-butylamine nBA, ammonium acetate AA, sulfide ion S 2- , Chloride ion Cl - , bromide ion Br - , iodide ion I - , Sulfate ion SO 4 2- , Thiocyanate ion SCN- , glutathione GSH and hydrazine, and their concentrations are all 50 μM. All test conditions are completed in ethanol and water (3:7, V / V) containing 5mM phosphate buffered saline (PBS), pH 7.4 system, and all spectra are at 25°C after the addition of analyte for 15min test the result. Pipette 25μL of the probe stock solution (1mM) into a 5mL colorimetric tube, then add 1.5mL ethanol, 250μL of 100mMPBS, and then pipette 25μL of the above analyte stock solution (10mM) into the colorimetric tube, and then set it with ultrapure water. Make up to 5mL. Shake well, let it stand for 15min, then measure. The result is as figure 2 shown.
[0036] from fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com