Uric acid reducing composition and preparation thereof
A uric acid-lowering technology and composition, applied in the field of uric acid-lowering composition and its preparations, can solve problems such as neutropenia, patient pain, and threats to health, and achieve the goal of reducing blood uric acid levels, good uric acid-lowering effects, and promoting uric acid excretion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 uric acid lowering test
[0045] 1. Materials and methods
[0046] Experimental animals: male SD rats of SPF grade, weighing 180±10 g, were provided by Guangdong Medical Experimental Animal Center (permit number: SYXK (Guangdong) 2013-0002).
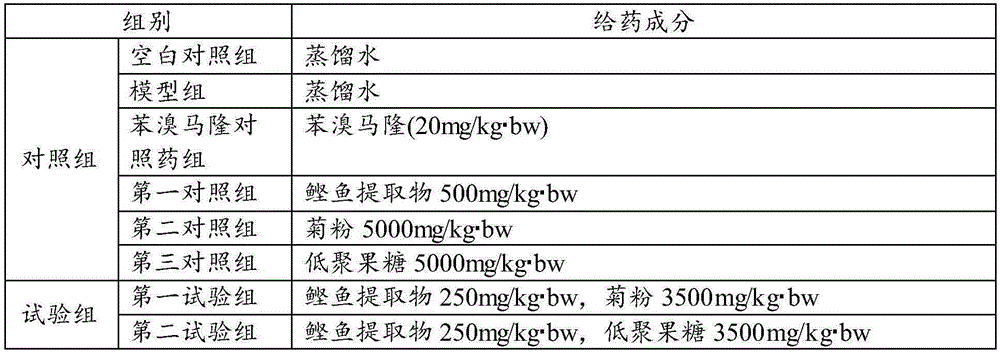
[0047] Test group: The test is divided into blank control group, model group, benzbromarone control group, first control group, second control group, third control group, first test group, and second test group, with 10 animals in each group rat.
[0048] Administration route: intragastric administration (consistent with the intended clinical administration route for humans). Administration volume: 10ml / kg. Dosing time: Gastrointestinal administration of therapeutic drugs at the same time as modeling, and intragastric administration according to body weight once a day in the morning. Administration period: continuous administration for 28 days.
[0049] Drug preparation: ready-to-use and ready-to-use, weigh a cer...
Embodiment 2
[0073] Embodiment 2 lowering uric acid test
[0074] 1. Materials and methods
[0075] Experimental animals: male SD rats of SPF grade, weighing 180±10 g, were provided by Guangdong Medical Experimental Animal Center (permit number: SYXK (Guangdong) 2013-0002).
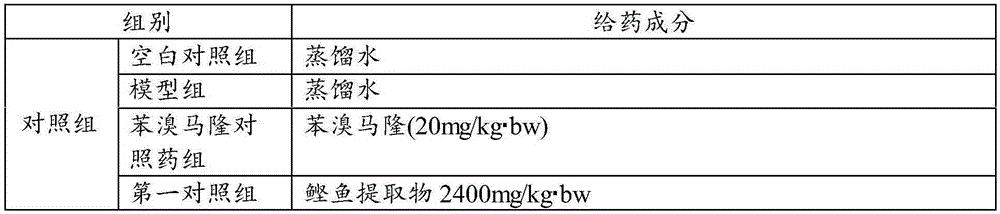
[0076] Test group: The test is divided into blank control group, model group, benzbromarone control group, first control group, second control group, third control group, first test group, and second test group, with 10 animals in each group rat.
[0077] Administration route: intragastric administration (consistent with the intended clinical administration route for humans). Administration volume: 10ml / kg. Dosing time: Gastrointestinal administration of therapeutic drugs at the same time as modeling, and intragastric administration according to body weight once a day in the morning. Administration period: continuous administration for 28 days.
[0078] Drug preparation: ready-to-use and ready-to-use, weigh a cer...
Embodiment 3
[0104] Embodiment 3 lowering uric acid test
[0105] 1. Materials and methods
[0106] Experimental animals: male SD rats of SPF grade, weighing 180±10 g, were provided by Guangdong Medical Experimental Animal Center (permit number: SYXK (Guangdong) 2013-0002).
[0107] Test groups: The test was divided into blank control group, model group, benzbromarone control group, first control group to sixth control group, first test group to sixth test group, with 10 rats in each group.
[0108] Administration route: intragastric administration (consistent with the intended clinical administration route for humans). Administration volume: 1.5ml / 100g. Dosing time: Gastrointestinal administration of therapeutic drugs at the same time as modeling, and intragastric administration according to body weight once a day in the morning. Administration period: continuous administration for 28 days.
[0109] Drug preparation: ready-to-use and ready-to-use, weigh a certain amount of the test su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com