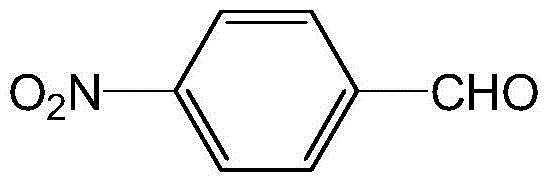
Preparation method of p-nitrobenzaldehyde
A technology of p-nitrobenzaldehyde and p-nitrobenzyl alcohol, applied in the field of preparation of known compounds, can solve the problems of no obvious advantages in production cost and industrial operation, expensive catalyst, difficult recovery and regeneration, etc. The effect of industrial preparation reaction operation safety, reduction of industrial preparation costs, and reduction of organic by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
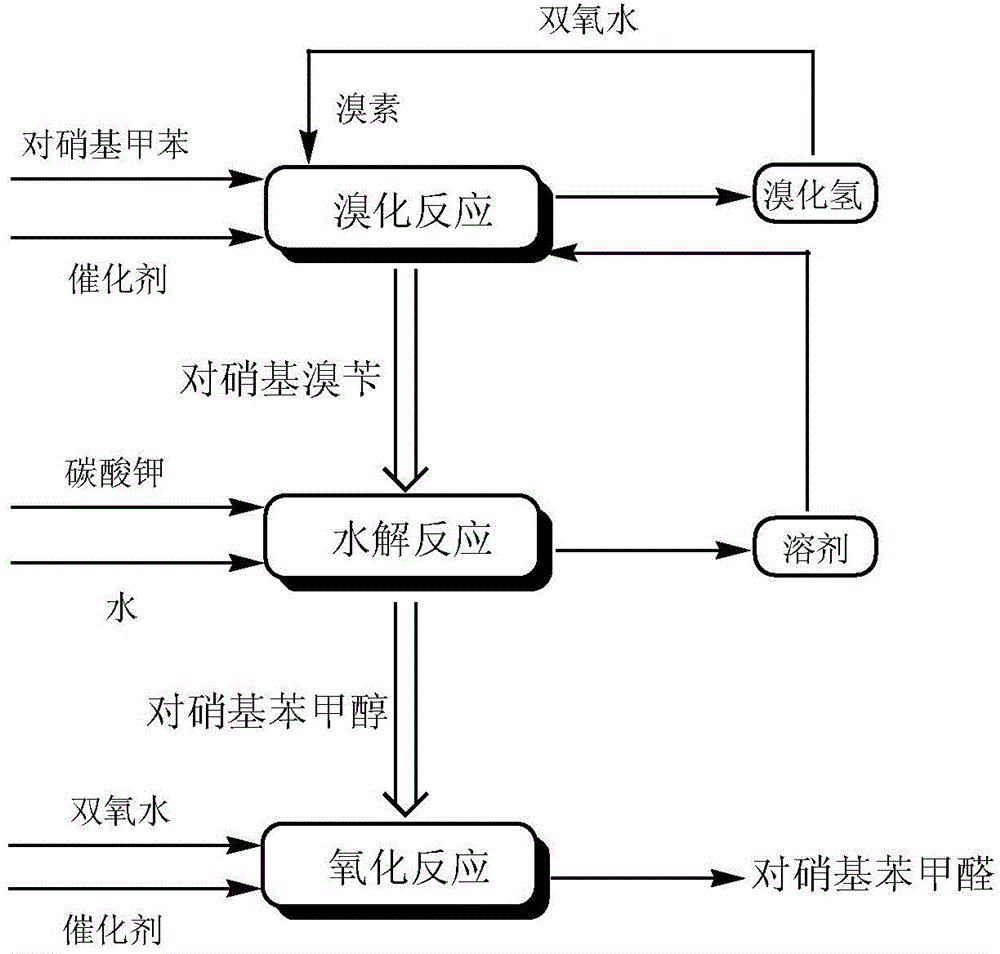
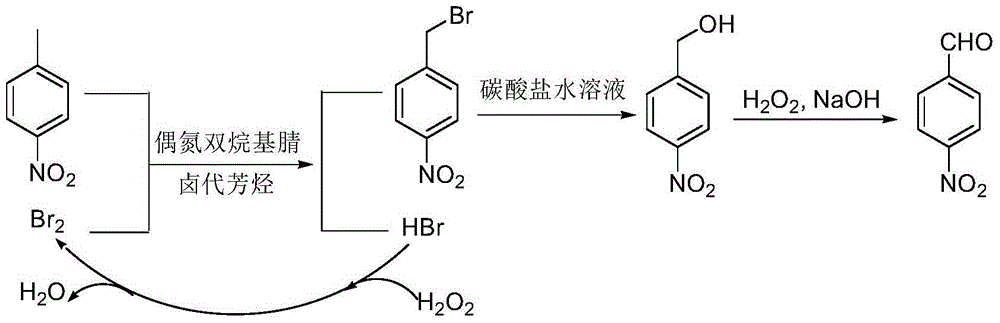
[0027] In the reactor, add 80g chlorobenzene, 13.7g p-nitrotoluene, 2.7g azobisisovaleronitrile, slowly add 9.6g bromine dropwise within 30min at 50°C, after the dropwise addition, stir and heat to reflux React until the bromine color fades. Slowly add 7.0 g of 27.5% hydrogen peroxide dropwise within 30 min, and stir and reflux for 3 h. Add 68.5g of 20% potassium carbonate aqueous solution to the reaction solution, stir and reflux for 30h, recover chlorobenzene by distillation, cool and stand to separate the water phase and the organic phase containing p-nitrobenzyl alcohol. Transfer the organic phase into the reactor, add 2.7g of sodium hydroxide, stir and control the temperature at 25°C, add 48.7g of 35% hydrogen peroxide dropwise within 30min, and heat to reflux for 26h. After the reaction was completed, it was cooled to room temperature, and the organic phase was separated by standing. After washing, the organic solvent was concentrated, and 11.5 g of p-nitrobenzaldehyde ...
Embodiment 2
[0029] In the reactor, add 80g of chlorobenzene, 13.7g of p-nitrotoluene, and 1.2g of azobisisoheptanonitrile, and slowly add 9.0g of bromine dropwise within 30min at 45°C. After the dropwise addition, stir and heat to reflux React until the bromine color fades. Slowly add 7.0 g of 27.5% hydrogen peroxide dropwise within 30 min, and stir and reflux for 2 h. Add 41.5 g of 30% potassium carbonate aqueous solution to the reaction solution, stir and reflux for 21 h, recover chlorobenzene by distillation, and cool and stand to separate the water phase and the organic phase containing p-nitrobenzyl alcohol. Transfer the organic phase into the reactor, add 1.5g of sodium hydroxide, stir and control the temperature at 30°C, add 62.0g of 27.5% hydrogen peroxide dropwise within 30min, and heat to reflux for 26h. After the reaction was completed, cool to room temperature, stand to separate the organic phase, concentrate the organic solvent after washing, and refine with ethanol to obtai...
Embodiment 3
[0031]In the reactor, add 80g of o-dichlorobenzene, 13.7g of p-nitrotoluene, and 1.2g of azobisisooctanonitrile, and slowly add 9.0g of bromine dropwise within 30min at 45°C. After the dropwise addition, stir and heat The reaction was refluxed until the color of bromine faded. Slowly add 7.0 g of 27.5% hydrogen peroxide dropwise within 30 min, and stir and reflux for 2 h. Add 41.5 g of 30% potassium carbonate aqueous solution to the reaction solution, stir and reflux for 23 hours, recover o-dichlorobenzene by distillation, and cool and stand to separate the water phase and the organic phase containing p-nitrobenzyl alcohol. Transfer the organic phase into the reactor, add 1.5g of sodium hydroxide, stir and control the temperature at 30°C, add 34.1g of 50% hydrogen peroxide dropwise within 30min, and heat to reflux for 26h. After the reaction was completed, cool to room temperature, stand to separate the organic phase, concentrate the organic solvent after washing, and refine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com