Nano-drug simulating superoxide dismutase or catalase and preparation method and application thereof
A catalase and nano-drug technology, applied in the application of oxidative stress-related diseases, in the field of nano-drug composition, can solve the problems of complex synthesis process and high cost of drugs or carrier materials, and ensure feasibility and safety. Safety, Guaranteed Safety, Ease of Removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
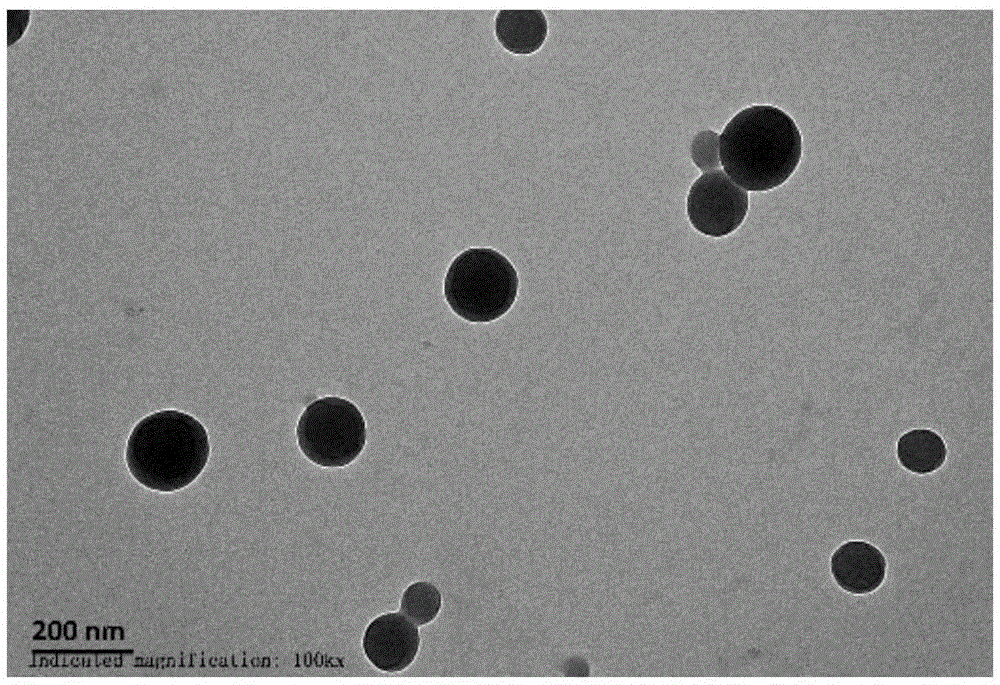
[0036]First, 1 mg of lecithin and 9 mg of polyethylene glycol-distearoyl phosphatidyl ethanolamine (wherein the molecular weight of polyvinyl alcohol is 1000 Da) was dissolved in 10 mL of double-distilled water under the condition of constant temperature magnetic stirring at 65 ° C, until the solid matter was completely dissolved, Let cool to room temperature. 30 mg of 4-hydroxymethylphenylboronic acid pinacol ester modified α-cyclodextrin derivative and 10 mg of 2,2,6,6-tetramethylpiperidine oxide were dissolved in 1.2 mL of methanol. The organic phase was then slowly added dropwise to the aqueous phase (1 mL / min) with stirring. After the dropwise addition was completed, stirring was continued at 25° C. for 2 h to volatilize and remove the organic solvent. The nanomedicine of the present invention can be obtained after centrifugal separation, washing with deionized water, and freeze-drying.
Embodiment 2
[0038] First, 4 mg of lecithin and 6 mg of polyethylene glycol-distearoyl phosphatidyl ethanolamine (wherein the molecular weight of polyvinyl alcohol is 2000 Da) was dissolved in 10 mL of double distilled water under the condition of constant temperature magnetic stirring at 65 ° C, until the solid matter was completely dissolved, Let cool to room temperature. 30 mg of 4-hydroxymethylbenzeneboronic acid pinacol ester modified α-cyclodextrin derivative and 10 mg of 4-hydroxy-2,2,6,6-tetramethylpiperidine oxide were dissolved in 1.2mL methanol / in acetonitrile. The organic phase was then slowly added dropwise to the aqueous phase (1 mL / min) with stirring. After the dropwise addition was completed, stirring was continued at 30° C. for 4 h to volatilize and remove the organic solvent. The nanomedicine of the present invention can be obtained after centrifugal separation, washing with deionized water, and freeze-drying.
Embodiment 3
[0040] First, 4 mg of lecithin and 6 mg of polyethylene glycol-distearoyl phosphatidyl ethanolamine (wherein the molecular weight of polyvinyl alcohol is 2000 Da) was dissolved in 10 mL of double distilled water under the condition of constant temperature magnetic stirring at 65 ° C, until the solid matter was completely dissolved, Let cool to room temperature. 30 mg of 4-hydroxymethylbenzeneboronic acid pinacol ester modified β-cyclodextrin derivative and 10 mg of 4-amino-2,2,6,6-tetramethylpiperidine oxide were dissolved in 1.2mL methanol / in dimethylformamide. The organic phase was then slowly added dropwise to the aqueous phase (1 mL / min) with stirring. After the dropwise addition was completed, stirring was continued at 60° C. for 8 h to volatilize and remove the organic solvent. The nanomedicine of the present invention can be obtained after centrifugal separation, washing with deionized water, and freeze-drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com