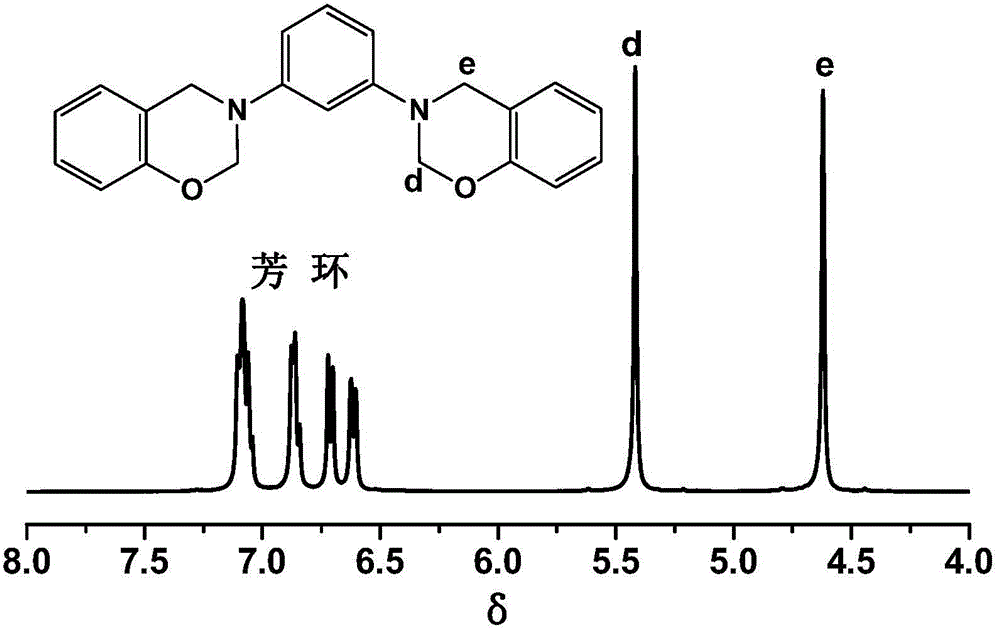
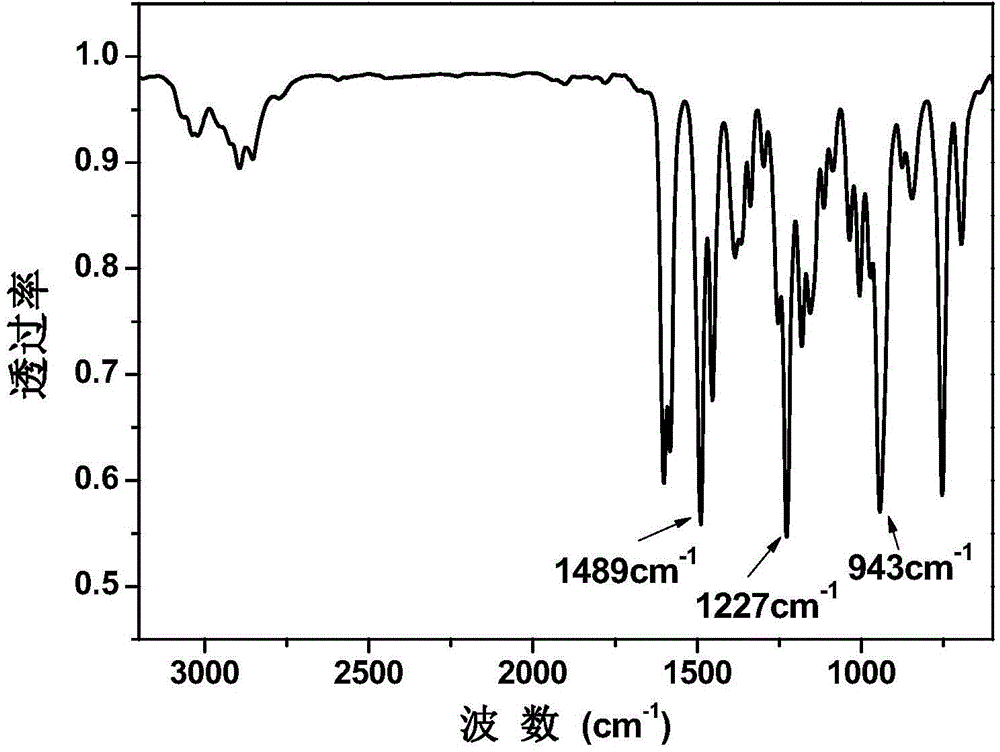
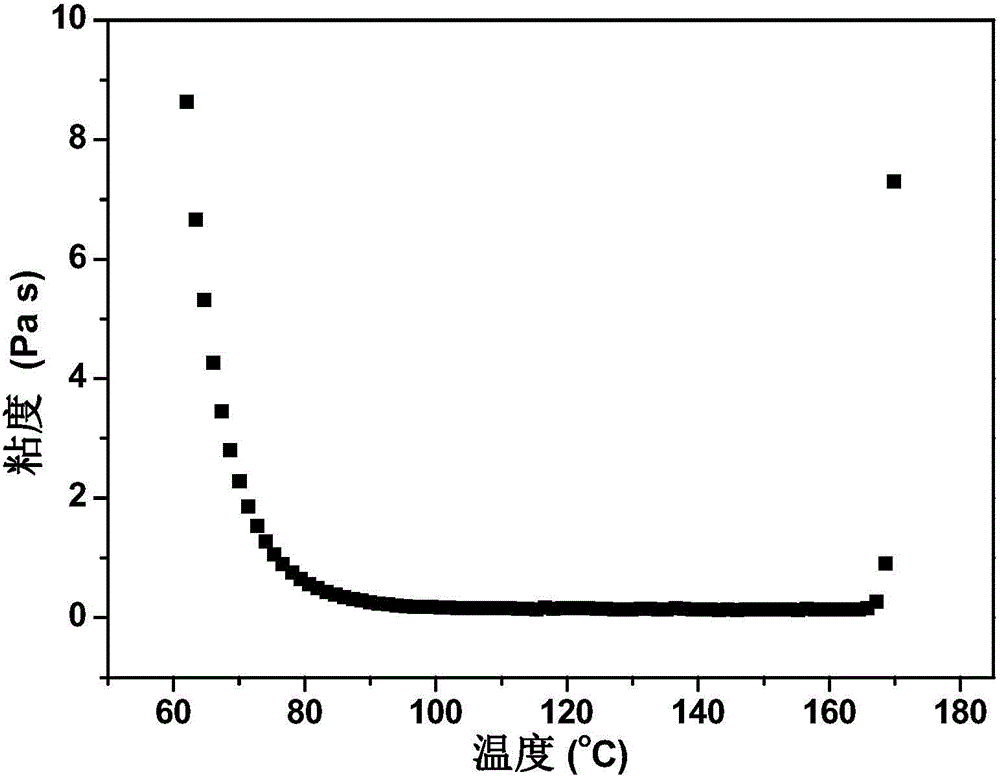
Aromatic diamine type benzoxazine resin and preparation method thereof
An aromatic diamine type, benzoxazine technology, applied in the field of thermosetting resins, to achieve the effects of good flame retardancy, high hydrogen bond density and high crosslinking density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add 20mmol m-phenylenediamine, 40mmol o-hydroxybenzaldehyde and 20mL absolute ethanol to a three-neck flask equipped with a magnet and a spherical condenser, react at 60°C for 0.5 hours, a large amount of yellow solid appears; after cooling to room temperature , add 20mmolNaBH 4 , reacted at 30°C for 1 hour, after cooling, pour the reaction solution into distilled water to precipitate, a large amount of white solid appeared, filtered, washed with distilled water, and dried in vacuum to obtain m-phenylenediamine-type Mannich base compound with a yield of 86%;
[0040] (2) Add 34mmol m-phenylenediamine type Mannich base compound and 100mL chloroform prepared by step (1) in the there-necked flask equipped with magneton and spherical condenser, then add 78mmol mass concentration of 37% to the dispersion Formaldehyde aqueous solution, stirred at room temperature for 6 hours, then continued to reflux for 12 hours; stop the reaction, remove the water phase after cooling, a...
Embodiment 2
[0045] (1) Add 20mmol m-phenylenediamine, 40mmol o-hydroxybenzaldehyde and 20mL absolute ethanol to a three-neck flask equipped with a magnet and a spherical condenser, react at 60°C for 0.5 hours, a large amount of yellow solid appears; after cooling to room temperature , add 40mmolNaBH 4 , react at 30°C for 1 hour, after cooling, pour the reaction solution into distilled water to precipitate, a large amount of white solid appears, filter, wash with distilled water, and dry in vacuum to obtain m-phenylenediamine-type Mannich base compound with a yield of 90%;
[0046] (2) add 34mmol m-phenylenediamine type Mannich base compound and 100mL dioxane prepared by 34mmol step (1) in the there-necked flask equipped with magneton and spherical condenser, then add 90mmol mass concentration to the dispersion liquid 37% formaldehyde aqueous solution, stirring at room temperature for 6 hours and then continuing to reflux for 12 hours; stop the reaction and remove the water phase after coo...
Embodiment 3
[0049] (1) Add 20mmol m-phenylenediamine, 40mmol o-hydroxybenzaldehyde and 20mL absolute ethanol to a three-neck flask equipped with a magnet and a spherical condenser, react at 60°C for 0.5 hours, a large amount of yellow solid appears; after cooling to room temperature , add 60mmolNaBH 4 , react at 30°C for 1 hour, after cooling, pour the reaction solution into distilled water to precipitate, a large amount of white solid appears, filter, wash with distilled water, and dry in vacuum to obtain m-phenylenediamine-type Mannich base compound with a yield of 91%;
[0050] (2) Add 34mmol m-phenylenediamine-type Mannich base compound and 100mL chloroform prepared by step (1) in the there-necked flask equipped with magneton and spherical condenser, then add 102mmol mass concentration of 37% to the dispersion Formaldehyde aqueous solution, stirred at room temperature for 6 hours, then continued to reflux for 12 hours; stop the reaction, remove the water phase after cooling, and remov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Storage modulus | aaaaa | aaaaa |
| Storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com