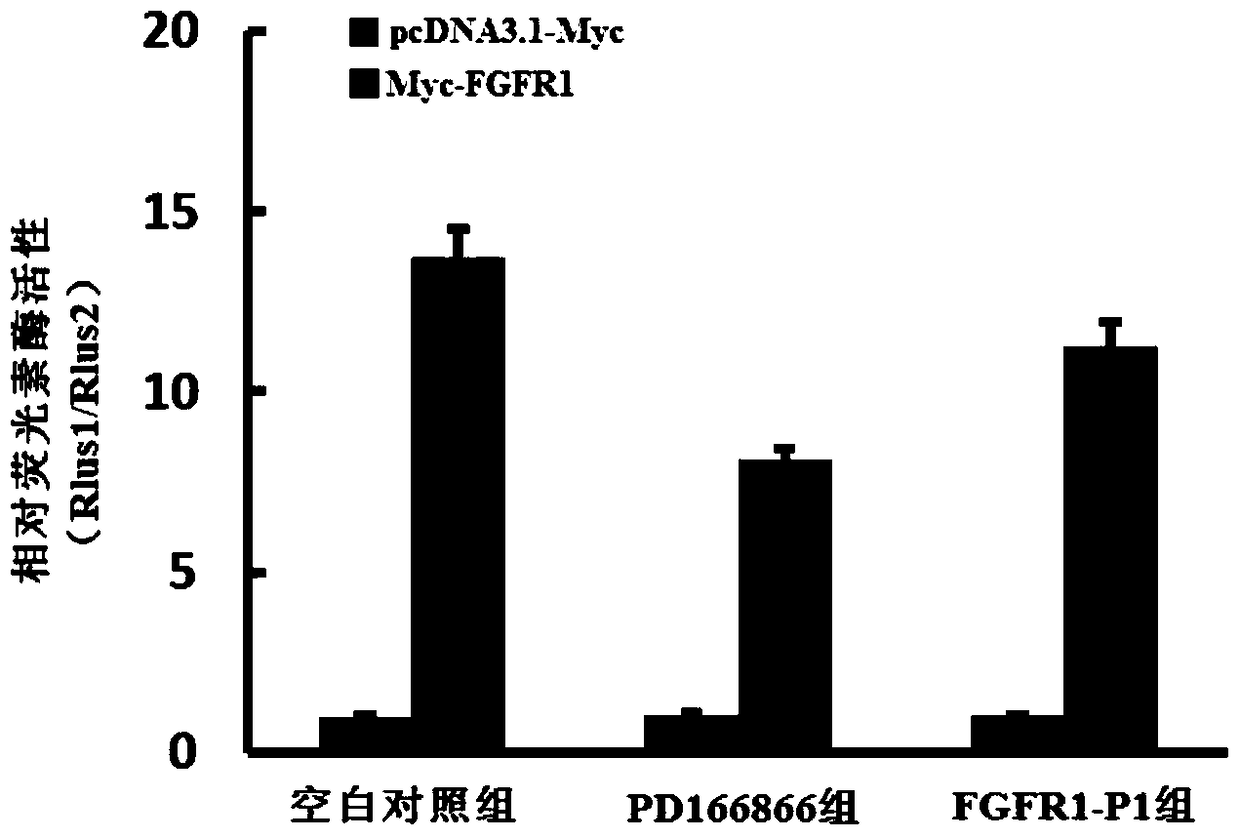
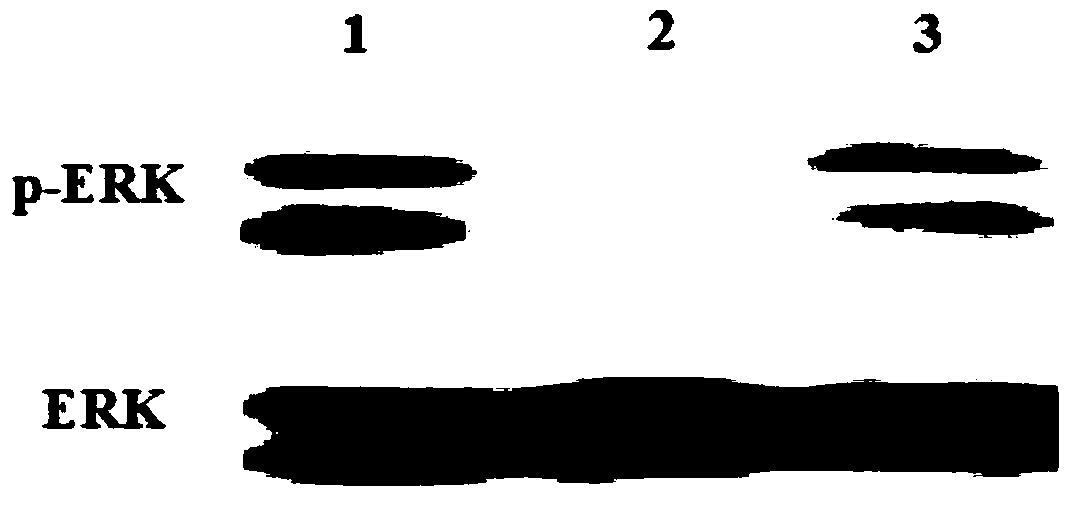
Polypeptides regulating fgfr1 activity and applications thereof
An active, R1-P1 technology, applied in the direction of peptides, non-central analgesics, bone diseases, etc., can solve the problems of not being able to meet the medical needs of patients, and achieve good application value, low immunogenicity, and small molecular weight.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1. Panning a polypeptide that can specifically bind to FGFR1 from a phage 12 peptide library
[0019] 1. Panning of phage 12 peptide library
[0020] Coat the microtiter plate with 150 μL of FGFR1 solution with a concentration of 20 μg / mL (sodium bicarbonate solution with a concentration of 0.1 mol / L and a pH of 8.6 as a solvent), coat at 4°C overnight, discard the coating solution, and fill the wells Add BSA solution with a concentration of 5 mg / mL (sodium bicarbonate solution with a concentration of 0.1 mol / L and a pH of 8.6 as a solvent), block at 37°C for 1 h, discard the blocking solution, and use TBST (that is, TBS containing Tween-20 buffer, the concentration of Tween-20 during the first round of panning was 1mL / L, and the concentration of Tween-20 was increased to 5mL / L during the second round and the third round of panning), washed 6 times, and dropped into phage (first round The mixture of phage 12 peptide library stock solution and TBST was put into ...
Embodiment 2
[0030] Example 2. Artificial synthesis of polypeptides that can specifically bind to FGFR1
[0031] According to the amino acid sequence of the R1-P1 peptide, Shanghai Qiangyao Biotechnology Co., Ltd. was entrusted to use the standard Fmoc protocol to synthesize the R1-P1 peptide in solid phase. It has been determined that the purity of the synthetic polypeptide is higher than 95%, and it is stored at -70°C.
Embodiment 3
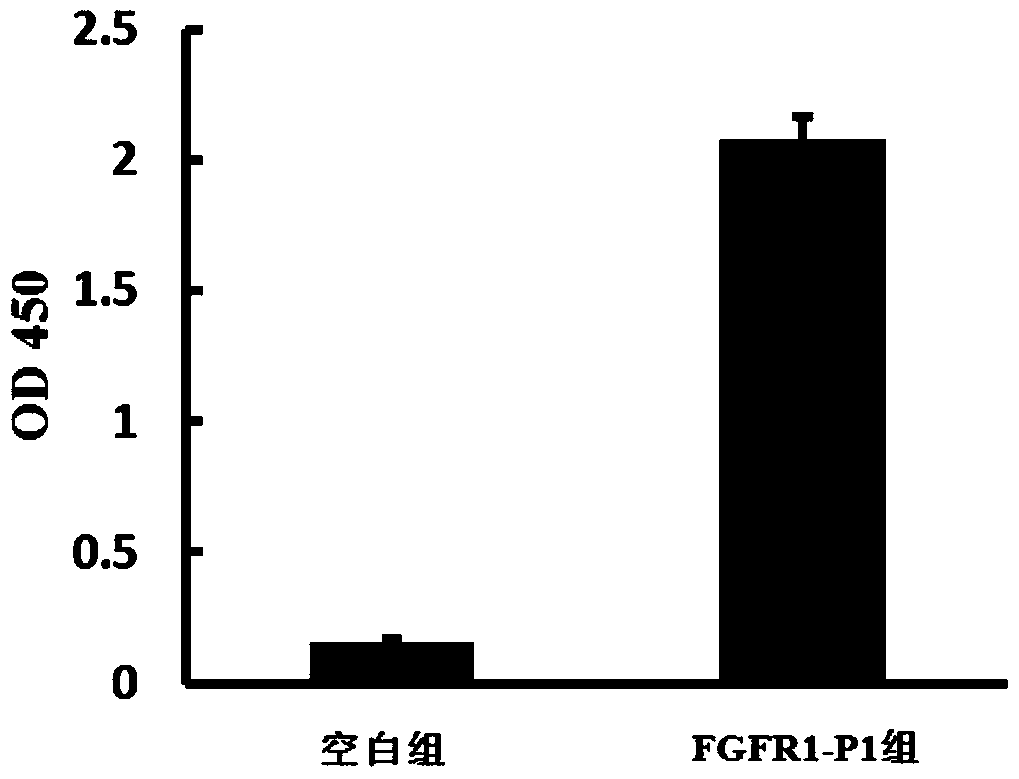
[0032] Example 3, ELISA method to verify the combination of synthetic polypeptide and FGFR1
[0033]In a 96-well ELISA plate, add 20 μL of R1-P1 peptide solution with a concentration of 1 mmol / μL and coating buffer (take 0.3 g of sodium carbonate, 0.58 g of sodium bicarbonate and 0.04 g of sodium azide, dissolve in water, Adjust the pH to 9.6, then add water to dilute to 200mL to obtain) 80μL, set up a blank control group (without adding R1-P1 peptide), coat overnight at 4°C, discard the solution, and add a concentration of 50g / L small Bovine serum solution (PBS with pH 7.4 as solvent), blocked at 37°C for 60 min, discarded the solution, washed 3 times with PBST (PBS containing Tween-20 at a concentration of 0.5 mL / L, pH 7.4), and added a concentration of 30 μL of 100 μg / mL FGFR1 solution and 70 μL of PBS with a pH of 7.4 were incubated at 37°C for 1 h, washed 3 times with PBST, and a 1:500 dilution of mouse anti-FGFR1 antibody was added (1% BSA solution with a mass percent co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com