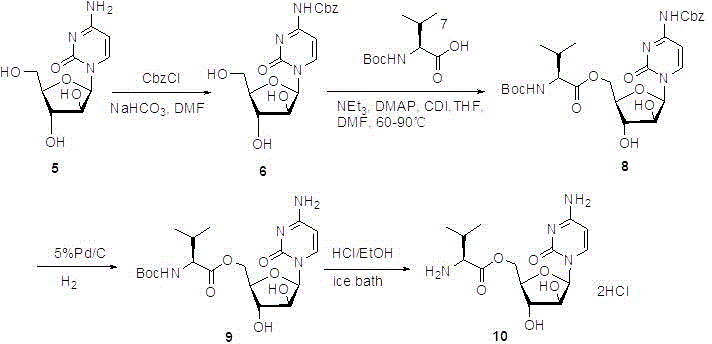
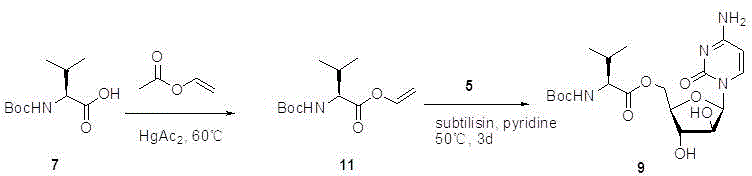
Preparation method of cytarabine 5'-O-L-valine ester hydrochloride
A technology of cytarabine ester and cytarabine, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of low selectivity, need column chromatography purification, small scale and the like, and achieves high selectivity, low cost and reaction yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] N 4 Preparation of -(N,N-dimethylaminomethylene)-cytarabine
[0022] Add 82mL of N,N-dimethylformamide dimethyl acetal (DMF-DMA) into a mixture of 60g of cytarabine and 240mL of ethanol, raise the temperature to 65°C for reaction, and the reaction ends after 4 hours. The reaction solution was lowered to room temperature, filtered, the solid was washed with methyl tert-butyl ether (MTBE), and dried under vacuum at 45°C to obtain N 4 -(N,N-Dimethylaminomethylene)-cytarabine ( N 4 -DMA-cytarabine) 69.5g, as a white solid, with a yield of 96% and a purity of 99.6% (HPLC).
Embodiment 2
[0024] 5′ -O -( N -tert-butoxycarbonyl-L-valine)-N 4 Preparation of -(N,N-dimethylaminomethylene)-cytarabine ester
[0025] Will 66.0g N 4 -DMA-Cytarabine was added to 660mL of dry DMF and stirred to dissolve, 124.3g of potassium valine was added and stirred at room temperature for 1 hour, the temperature was lowered to -10℃~-5℃, 48.2g of ethyl chloroformate was added dropwise, and After the addition, the temperature was gradually raised to 0°C to continue the reaction for 4 hours, and then gradually raised to room temperature for 2 hours, then 6.6L of isopropyl ether was added, stirred at 0°C for 2 hours, and filtered. Add 1.7L chloroform to the solid, wash with 15% sodium carbonate solution (300mLx2), saturated brine (300mL) successively, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 5' -O -( N -tert-butoxycarbonyl-L-valine)-N 4 -(N,N-dimethylaminomethylene)-cytarabine ester 97.2g, liquid phase purity 94%. The compound was detect...
Embodiment 3
[0027] Cytarabine 5'-O-L -Preparation of valine ester hydrochloride
[0028] will be 5' -O -( N -tert-butoxycarbonyl-L-valine)-N 4 -(N,N-Dimethylaminomethylene)-cytarabine ester 103g was added to 800mL isopropanol, stirred to dissolve, hydrogen chloride gas was passed into the reaction liquid until saturated, stirred overnight, and the solvent was evaporated under reduced pressure , add 800mL of acetonitrile to beat for 2 hours, filter, and blow dry at 45°C to obtain cytarabine 5'-O-L -Valine ester hydrochloride 64g, is white solid, yield 74.4%, liquid phase purity 97%.
[0029] Refined: Cytarabine 5'-O-L - 64g of valine ester hydrochloride was added with 640mL of 95% ethanol, heated at 50°C for beating, cooled to room temperature, and filtered to obtain 50g of product with a purity of 99%.
[0030] The compound was detected by mass spectrometry (ESI-MS) and nuclear impact resonance (HNMR). ESI-MS detection conditions are: m / z=343[M+H] + ,172[M+2H] ++ ,685[2M+H] + , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com