A method for preparing 5-hydroxymethylfurfural from glucose
A technology of hydroxymethylfurfural and glucose, which is applied in the field of 5-hydroxymethylfurfural, can solve the problems of high price of ionic liquid, unfavorable industrial production, high catalyst toxicity, etc., and achieve large-scale production, good economy and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
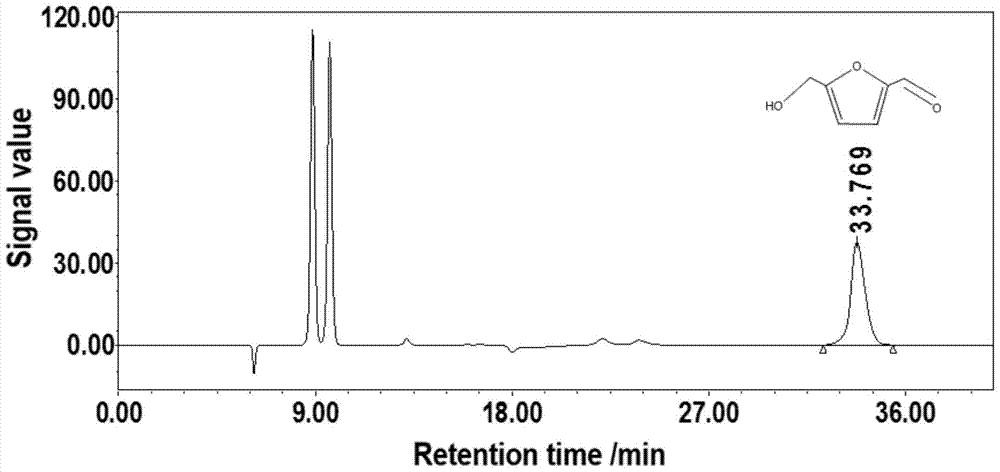
[0034] Add 50g of water, 2.5g of glucose, 3g of choline chloride and 0.3ml of hydrochloric acid (37%) into a 100mL round bottom flask, fully dissolve. The flask was placed in a water bath for heating reaction at a temperature of 100° C., and the reaction time was 4 h. After the reaction, the 5-hydroxymethylfurfural obtained by the reaction was extracted with acetonitrile, and the acetonitrile was separated by distillation under reduced pressure to obtain the product 5-hydroxymethylfurfural. The molar yield of the product was 0.86mol% as determined by HPLC.
Embodiment 2
[0036] Add 50g of water, 2.5g of glucose, 0.2g of choline, and 0.1g of borax into a 100mL round-bottomed flask to fully dissolve. The flask was placed in a water bath, and the heating reaction was carried out under N2 atmosphere, the temperature was 30° C., and the reaction time was 20 h. After the reaction was completed, water was distilled off under reduced pressure. Add 0.3ml of hydrochloric acid (37%) to the residue to neutralize the reaction solution, then add 3g of choline chloride to mix, place in a water bath for heating reaction, the temperature is 90°C, and the reaction time is 6h. After the reaction, the 5-hydroxymethylfurfural obtained by the reaction was extracted with acetonitrile, and the acetonitrile was distilled and separated under reduced pressure to obtain the product 5-hydroxymethylfurfural. The molar yield of the product was 22.5 mol% as determined by HPLC.
Embodiment 3
[0038] Add 50g of water, 2.5g of glucose, 0.2g of choline, and 0.1g of borax into a 100mL round-bottomed flask to fully dissolve. The flask was placed in a water bath, and the heating reaction was carried out under N2 atmosphere at a temperature of 55° C., and the reaction time was 8 h. After the reaction was completed, water was distilled off under reduced pressure. Add 0.3ml of hydrochloric acid (37%) to the residue to neutralize the reaction solution, then add 3g of choline chloride to mix, place in a water bath for heating reaction, the temperature is 90°C, and the reaction time is 6h. After the reaction, the 5-hydroxymethylfurfural obtained by the reaction was extracted with acetonitrile, and the acetonitrile was separated by distillation under reduced pressure to obtain the product 5-hydroxymethylfurfural. The molar yield of the product was 20.4 mol% as determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com