Stable NEFA (non-esterified fatty acid) measuring kit
A free fatty acid and kit technology, applied in the field of medical testing, can solve the problems of short storage time, inaccurate test results, and unsuitable for large-scale application, achieve accurate and reliable test data, improve analytical sensitivity, and ensure long-term high activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] One, a stable enzymatic free fatty acid assay kit, the kit is made up of liquid double reagents prepared according to the following ingredients and ratios, wherein:
[0050] Reagent 1:
[0051]
[0052] Reagent 2:
[0053]
[0054]
[0055] The volume ratio of the reagent 1 and reagent 2 is 4:1.
[0056] 2. Operation method
[0057] The free fatty acid assay kit described in this example uses an automatic biochemical analyzer, taking Hitachi 7080 as an example, and the specific operations are as follows:
[0058] Table 1 Detection method of the kit of the present invention
[0059]
[0060] Test conditions: 37°C, the optical path of the cuvette is 1.0cm, the main detection wavelength is 546nm, and the secondary wavelength is 660nm. The free fatty acid content was calculated according to the following formula.
[0061]
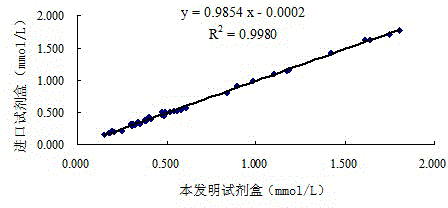
[0062] 3. The correlation between the kit of the present invention and the imported enzyme method kit
[0063] Using the kit descri...
Embodiment 2
[0096] One, a stable enzymatic free fatty acid assay kit, the kit is made up of liquid double reagents prepared according to the following ingredients and ratios, wherein:
[0097] Reagent 1:
[0098]
[0099] Reagent 2:
[0100]
[0101]
[0102] The volume ratio of the reagent 1 and reagent 2 is 4:1.
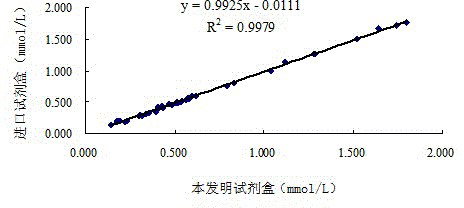
[0103] 2. The correlation between the kit of the present invention and the imported kit
[0104] Using the kit described in this example, according to the method described in Example 1, and simultaneously with the commercially available imported kit (Desay) to measure the free fatty acid content in 40 cases of human serum samples, the imported kit was operated according to its instructions , The measurement results are shown in Table 6 below. In the following table, the reference range of the imported kit is 0.10-0.60mmol / L, and the reference range of the kit of the present invention is 0.13-0.77mmol / L.
[0105] Table 6 The assay results of the kit of the present ...
Embodiment 3
[0133] One, a stable enzymatic free fatty acid assay kit, the kit is made up of liquid double reagents prepared according to the following ingredients and ratios, wherein:
[0134] Reagent 1:
[0135]
[0136] Reagent 2:
[0137]
[0138]
[0139] The volume ratio of the reagent 1 and reagent 2 is 4:1.
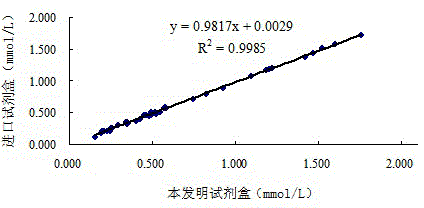
[0140] 2. The correlation between the kit of the present invention and the imported kit
[0141] Using the kit described in this example, according to the method described in Example 1, the free fatty acid content in 40 cases of human serum samples was measured using a commercially available imported kit (Desay) at the same time, and the imported kit was operated according to its instructions , The measurement results are shown in Table 10 below. In the following table, the reference range of the imported kit is 0.10-0.60mmol / L, and the reference range of the kit of the present invention is 0.13-0.77mmol / L.
[0142] Table 10 The test kit of the present invention a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com