Thiooxacycloalkane compound and synthetic method therefor
A technology for thiooxane and oxane, which is applied in the field of thiooxane compounds and their synthesis, and achieves the effects of easy availability, environmental friendliness and low toxicity of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
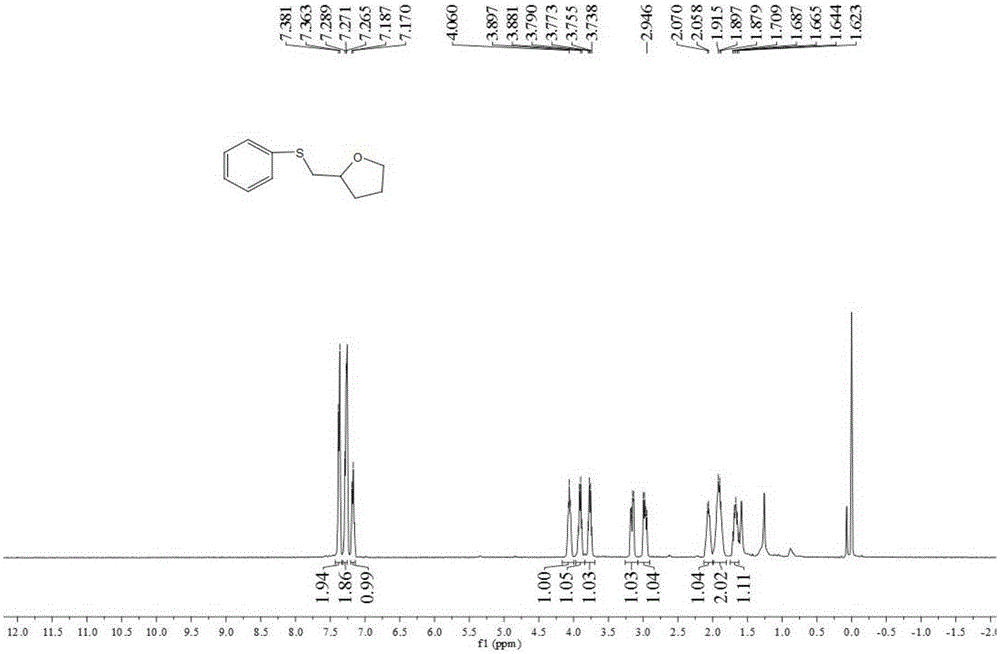
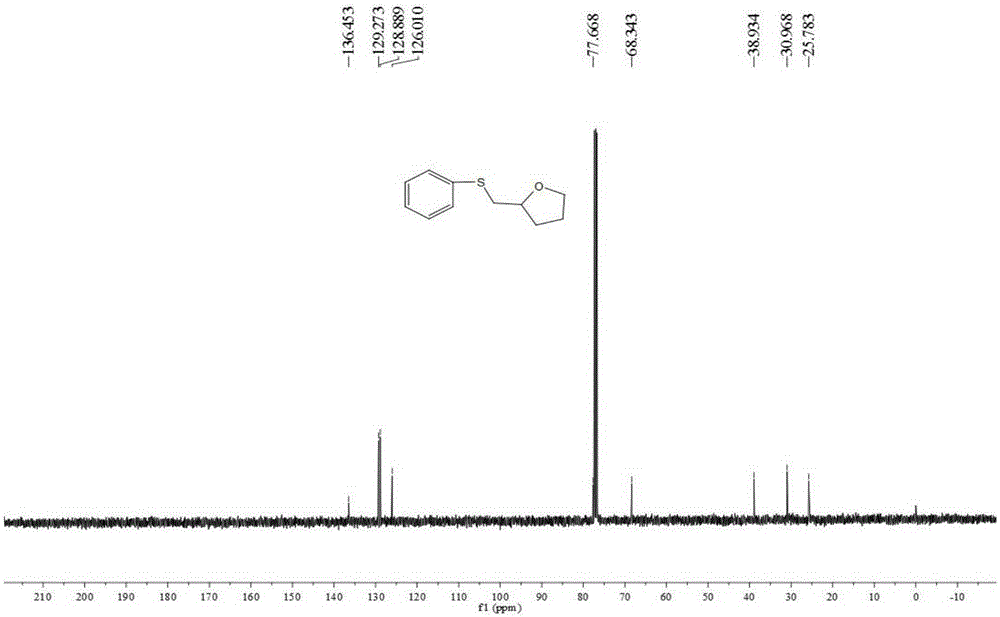
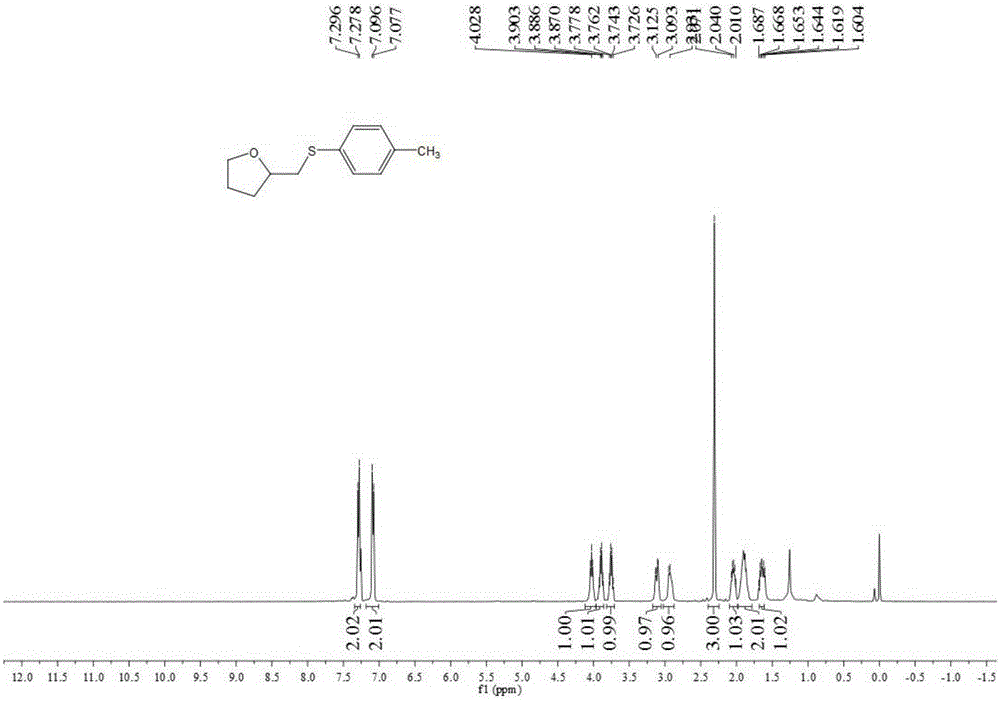
[0038] Add 0.25 mmol of 4-penten-1-ol, 0.25 mmol of sodium p-toluenesulfinate, 0.025 mmol of cuprous chloride, and 2 ml of acetonitrile into a screw-top test tube, and stir and react at 80°C for 5 hours , stop heating and stirring, and cool to room temperature. The reaction material was decompressed and rotary evaporated to remove the solvent, and then separated and purified by column chromatography to obtain a thioxacycloalkane compound. The column chromatography eluent used was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 3:1 , yield 85%.
Embodiment 2
[0040] Add 0.25 mmol of 4-penten-1-ol, 0.30 mmol of sodium p-toluenesulfinate, 0.025 mmol of cuprous chloride, and 2 ml of acetonitrile into a screw-top test tube, and stir and react at 90°C for 30 hours , stop heating and stirring, and cool to room temperature. The reaction material is decompressed and rotary evaporated to remove the solvent, and then separated and purified by column chromatography to obtain a thioxacycloalkane compound. The column chromatography eluent used is a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 1:1 , yield 81%.
Embodiment 3
[0042] Add 0.25 mmol of 4-penten-1-ol, 0.40 mmol of sodium p-toluenesulfinate, 0.020 mmol of cuprous chloride, and 2 ml of toluene into a screw-top test tube, and stir and react at 80°C for 6 hours , stop heating and stirring, and cool to room temperature. The reaction material was decompressed and rotary evaporated to remove the solvent, and then separated and purified by column chromatography to obtain a thioxacycloalkane compound. The column chromatography eluent used was a mixed solvent of petroleum ether: ethyl acetate with a volume ratio of 5:1 , yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com