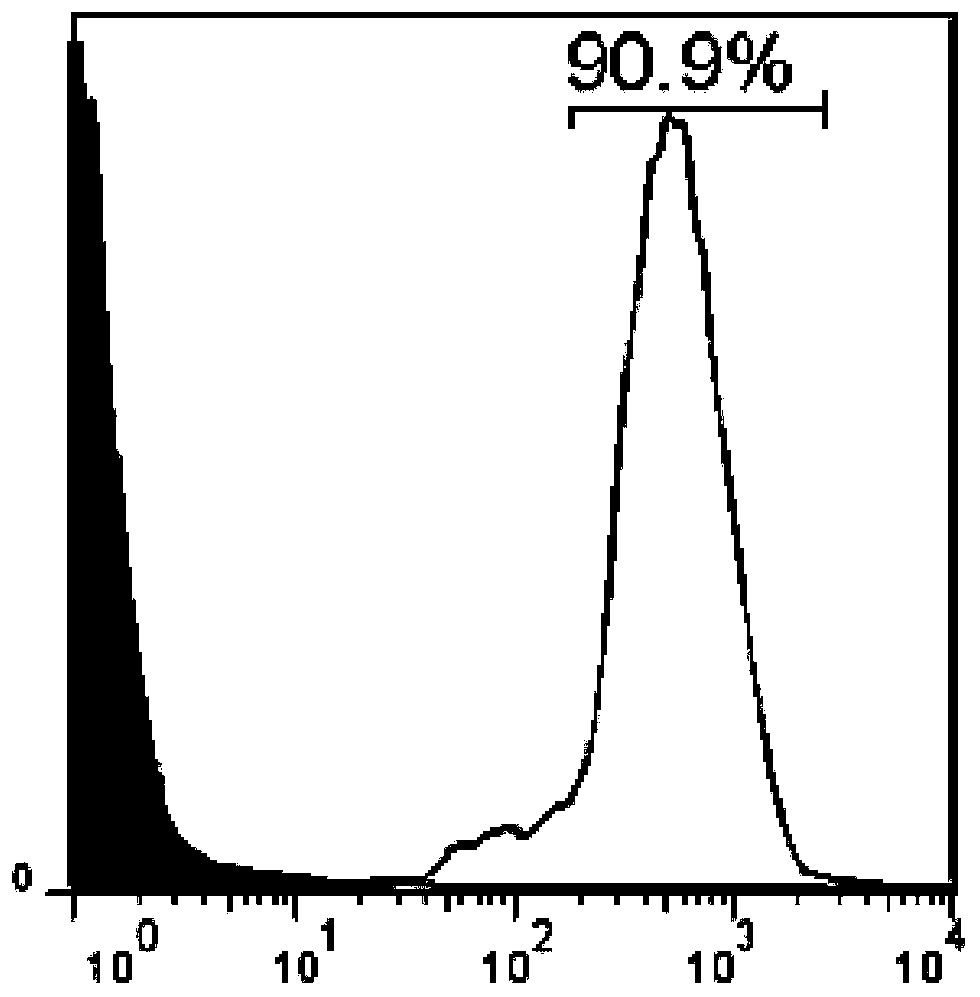
Fitc-labeled vitamin b12 derivatives and their synthesis and application
A VB12-FITC, vitamin technology, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of high cost of vitamin B12 imaging and difficult operation, and achieve good application prospects and low toxicity of raw materials. , the effect of mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
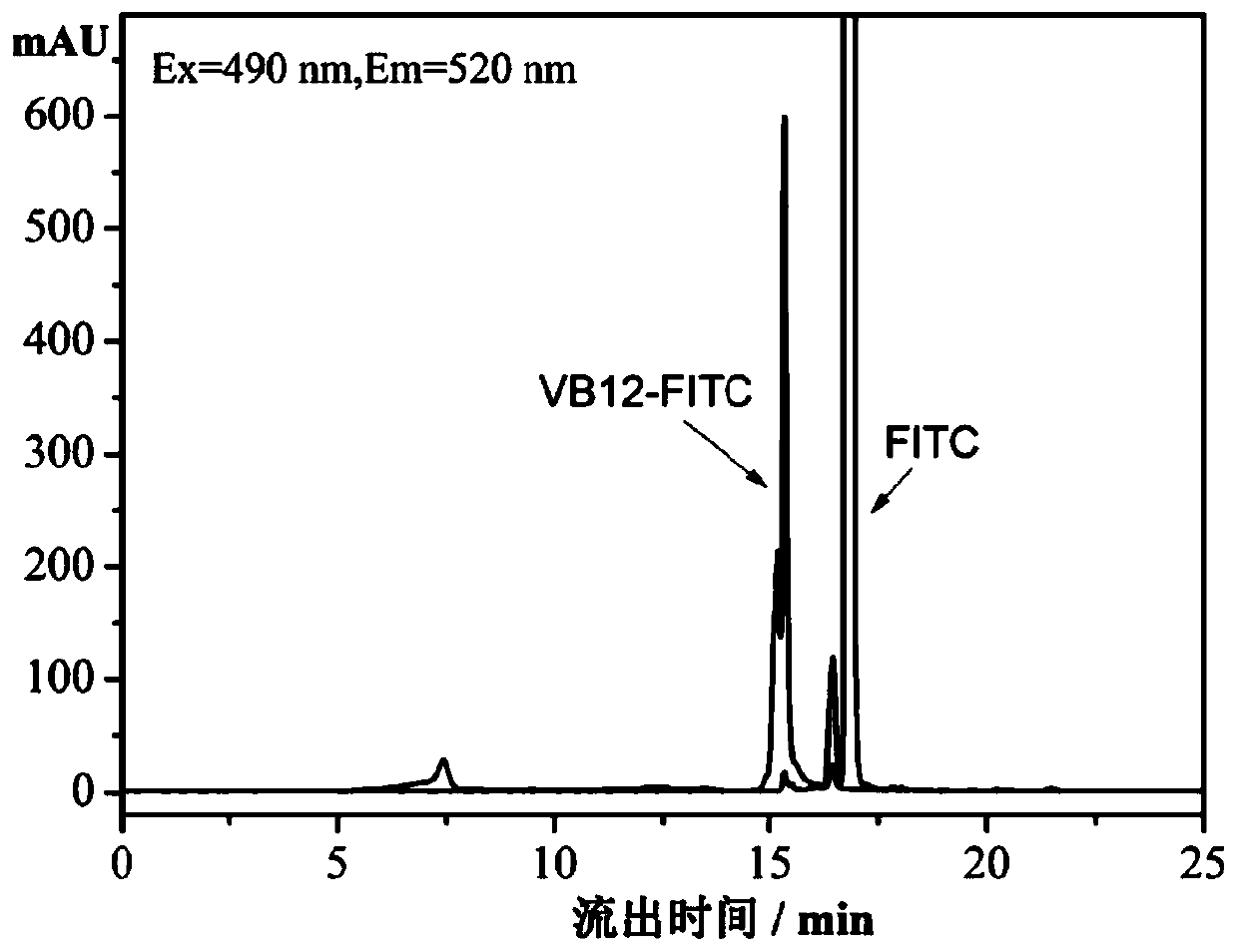
[0029] Embodiment 1: Preparation of VB12-FITC according to the present invention
[0030] (A) Dissolve 200mg of vitamin B12 in 4ml of dehydrated dimethyl sulfoxide (DMSO) under nitrogen protection, add 200mg of activator N,N'-carbonyldiimidazole (CDI), and protect from light at 20°C After activation for 12 hours, 400 mg of di-1,8-amino-3,6-dioxahexane was added, and the reaction was continued at 20° C. for 6 hours. Precipitate the reaction product with 500ml of acetone, centrifuge at 10000r / min for 10min to separate the reaction product, and dry it in vacuum at 20°C to obtain aminated vitamin B12.
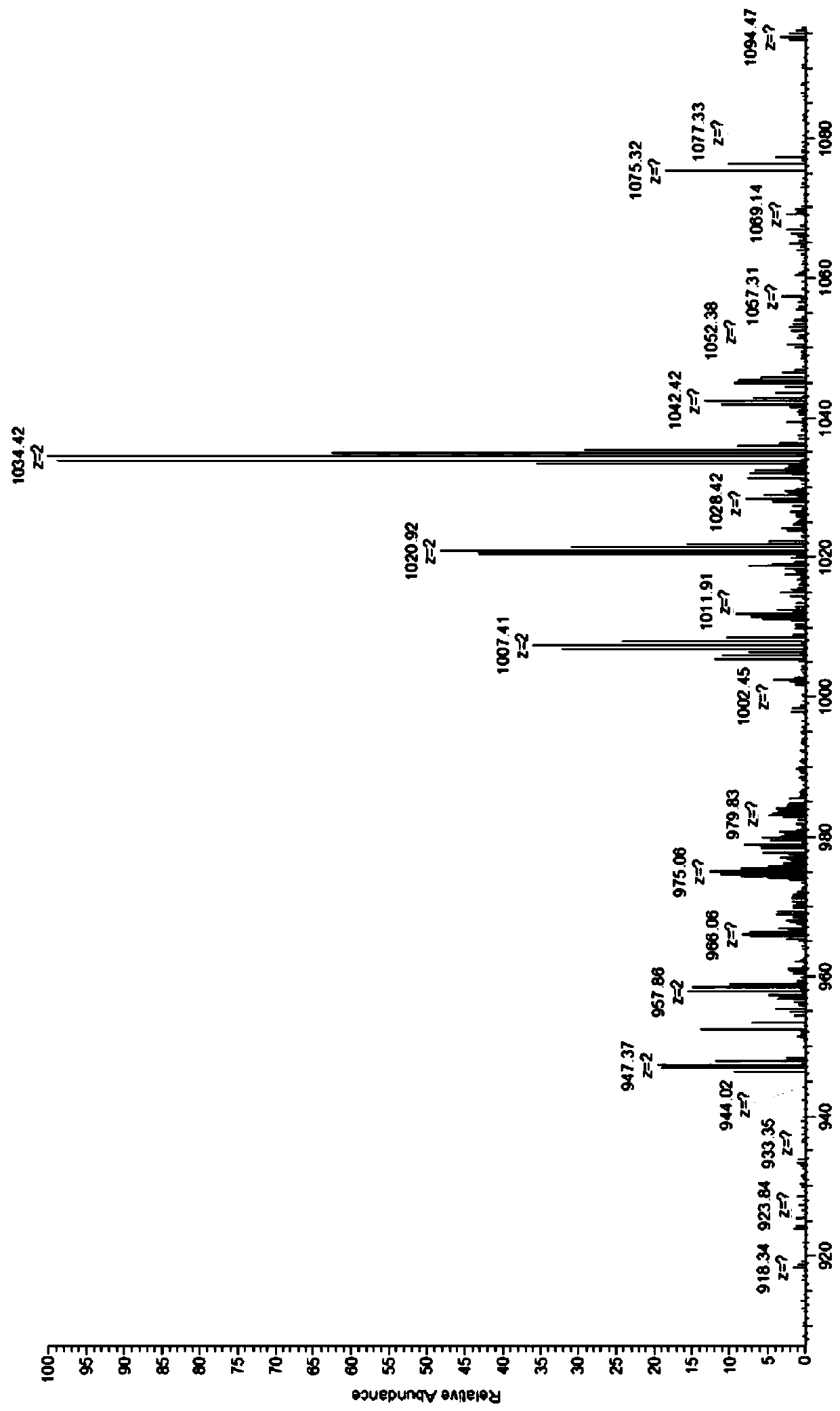
[0031] (B) Weigh 150ng of the aminated vitamin B12 obtained in step A and dissolve it in 4ml of dimethyl sulfoxide (DMSO) that has been treated with water removal, add 0.1ml of catalyst triethylamine, and add FITC in a ratio of 1:1, After reacting in the dark at 20°C for 6 hours, the reaction product was precipitated and separated, and vacuum-dried at 20°C to obtain FITC-labeled v...
Embodiment 2
[0032] Embodiment 2: the preparation of VB12-FITC described in the present invention
[0033] (A) Dissolve 200mg of vitamin B12 in 4ml of dehydrated dimethyl sulfoxide (DMSO) under nitrogen protection, add 300mg of activator N,N'-carbonyldiimidazole (CDI), and protect from light at 22°C After activation for 12 hours, 600 mg of 1,8-diamino-3,6-dioxahexane was added, and the reaction was continued at 22° C. for 6 hours. The reaction product was precipitated with 500 ml of acetone, centrifuged at 10,000 r / min for 10 min to separate the reaction product, and vacuum-dried at 22° C. to obtain aminated vitamin B12.
[0034] (B) Weigh 150ng of the aminated vitamin B12 obtained in step A and dissolve it in 4ml of dimethyl sulfoxide (DMSO) that has been treated with water removal, add 0.1ml of catalyst triethylamine, and add FITC at a ratio of 1:1.25, After reacting in the dark at 22°C for 6 hours, the reaction product was precipitated and separated, and vacuum-dried at 22°C to obtain ...
Embodiment 3
[0035] Embodiment 3: the preparation of VB12-FITC described in the present invention
[0036] (A) Dissolve 200mg of vitamin B12 in 4ml of dehydrated dimethyl sulfoxide (DMSO) under nitrogen protection, add 400mg of activator N,N'-carbonyldiimidazole (CDI), and protect from light at 25°C After activation for 12 hours, 800 mg of di-1,8-amino-3,6-dioxahexane was added, and the reaction was continued at 25° C. for 6 hours. Precipitate the reaction product with 500ml of acetone, centrifuge at 10000r / min for 10min to separate the reaction product, and vacuum dry at 20°C to obtain aminated vitamin B12.
[0037] (B) Weigh 150ng of the aminated vitamin B12 obtained in step A and dissolve it in 4ml of dimethyl sulfoxide (DMSO) that has been treated with water removal, add 0.1ml of catalyst triethylamine, and add FITC at a ratio of 1:1.5, After reacting in the dark at 25°C for 6 hours, the reaction product was precipitated and separated, and vacuum-dried at 20°C to obtain FITC-labeled v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com