Efficient photocatalytic-water-splitting hydrogen production catalyst and preparation method thereof
A technology of photocatalysis and catalyst, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, hydrogen production, etc., can solve the problem of low efficiency of photocatalytic decomposition of water to produce hydrogen, meet the requirements of industrialization, improve efficiency, and increase speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Get 0.4689 grams of copper nitrate and put it into a 250 milliliter beaker and stir for 10 minutes, then add 100 milliliters of sodium hydroxide solution of 0.25 moles per liter under continuous stirring, and continue to stir for 2 hours;
[0027] (2) Wash and centrifuge the suspension in step (1) 3 times with deionized water, wash and centrifuge 2 times with ethanol, pour off the supernatant and put it into an oven at 80 degrees Celsius to dry for 12 hours;
[0028] (3) Grinding the dried product of step (2) and sodium hypophosphite (the quality of sodium hypophosphite: the quality of the dried product of step (2)=5:1) in a mortar;
[0029] (4) get the product of step (3) gained and put into the tubular furnace with nitrogen protection device and roast 1 hour under the condition of 300 degree;
[0030] (5) the product after step (4) roasting is washed with deionized water and centrifuged 3 times, and ethanol is washed and centrifuged 2 times to obtain the product c...
Embodiment 2
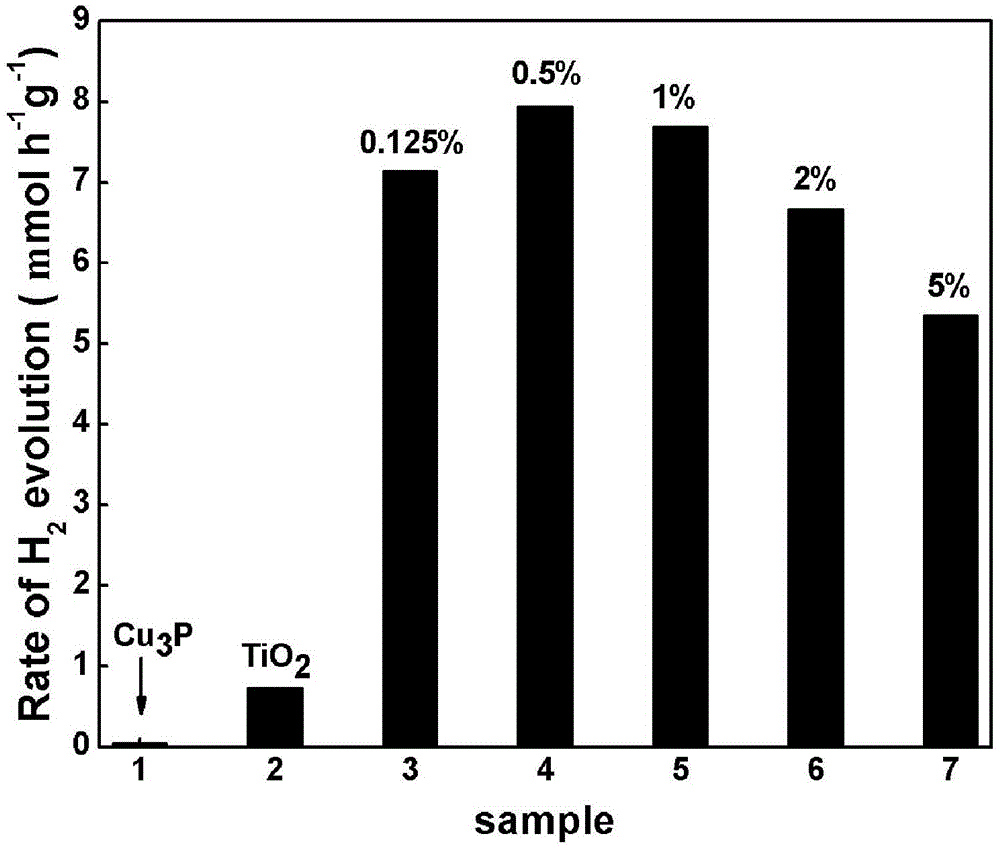
[0036] Step (1)~(8) is the same as embodiment 1, the product (pure phase titanium dioxide (TiO2) in step (8) 2 ) nanocrystals and the product (pure phase copper phosphide (Cu 3 P) The mass ratio is (mass of titanium dioxide: mass of copper phosphide = 100:0.125), grind in a mortar for 45 minutes and mix evenly to obtain a sample of 0.125wt% Cu 3 P / TiO 2 ; 0.1 gram of the catalyst, 90 milliliters of water, and 10 milliliters of triethanolamine with a mass fraction of 99.5% were mixed to form a uniform suspension, which was tested by the Beijing Pufferai Optical Hydrogen Production System and the Shimadzu GC-81 chromatograph. The photocatalytic water splitting rate of the photocatalytic water splitting agent prepared by the present invention is 7.149mmolh -1 g -1 , 0.74mmolh hydrogen production rate than pure titanium dioxide -1 g -1 There has been a significant improvement, and the improvement effect has exceeded 9 times.
Embodiment 3
[0038] Step (1)~(8) is the same as embodiment 1, the product (pure phase titanium dioxide (TiO2) in step (8) 2 ) nanocrystals and the product (pure phase copper phosphide (Cu 3 P) The mass ratio is (mass of titanium dioxide: mass of copper phosphide = 100:0.5), grind in a mortar for 45 minutes and mix evenly to obtain a sample of 0.5wt% Cu 3 P / TiO 2 ; 0.1 gram of the catalyst, 90 milliliters of water, and 10 milliliters of triethanolamine with a mass fraction of 99.5% were mixed to form a uniform suspension, which was tested by the Beijing Pufferai Optical Hydrogen Production System and the Shimadzu GC-81 chromatograph. The photocatalytic water splitting rate of the photocatalytic water splitting agent prepared by the present invention is 7.939mmolh -1 g -1 , 0.74mmolh hydrogen production rate than pure titanium dioxide -1 g -1 There has been a significant improvement, and the improvement effect has exceeded 10 times.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com