Conjugated polymer containing fluorene as well as preparation method and application thereof
A technology of conjugated polymer and fluorene ring, which is applied in the field of conjugated polymer containing fluorene ring and its preparation, can solve the problems of large cytotoxicity, poor photostability, limited development and the like, and achieves low cytotoxicity and good stability. , the effect of good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
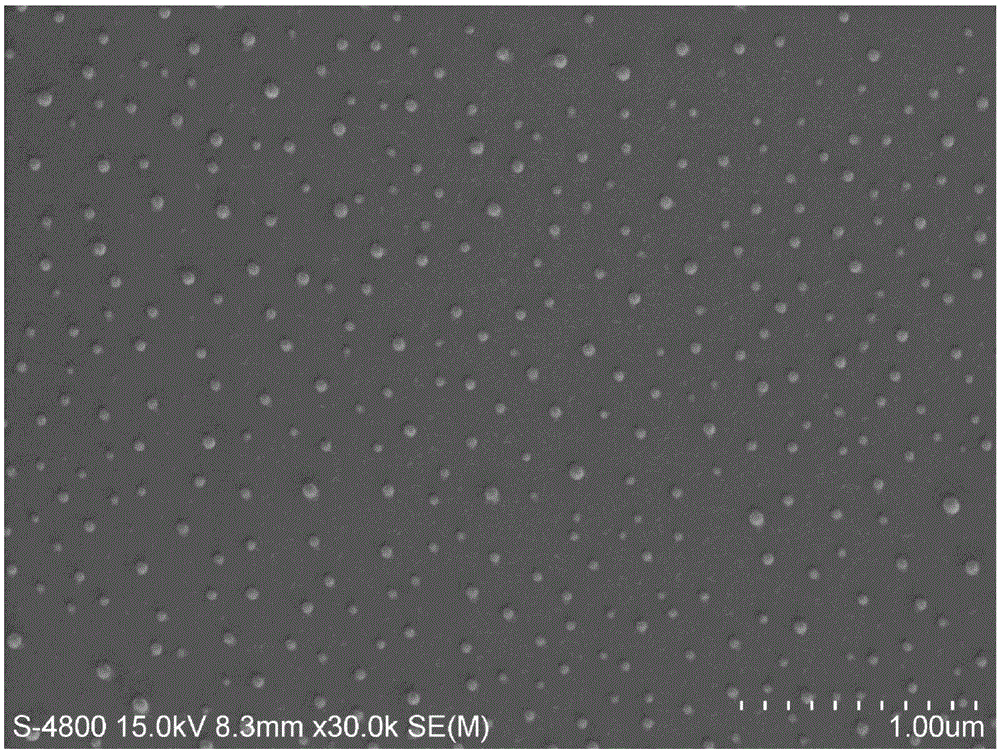
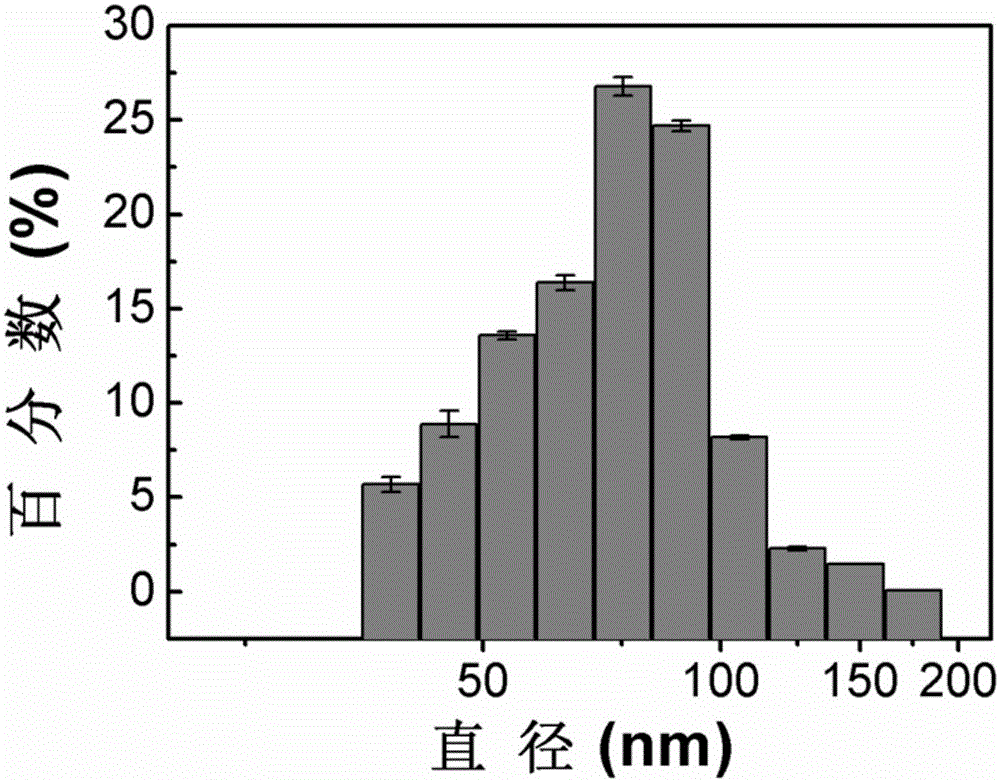
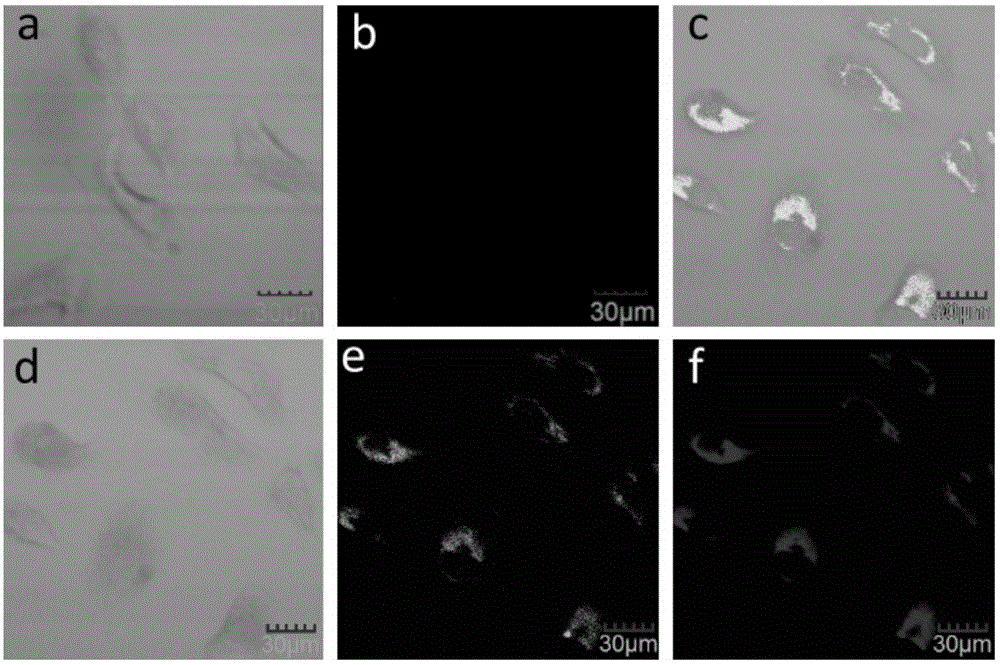
Image
Examples
Embodiment 1
[0024] 1) Add 2.68g (0.01mol) of 1,4-dibromohydroquinone and 7.65g (0.022mol) of p-toluenesulfonyl to a 250mL round bottom flask equipped with a reflux condenser tetraethylene glycol, 5.52g (0.04mol) of potassium carbonate and 150mL of acetone. The reaction was heated to reflux with stirring for 48 hours. Stop the reaction, cool to room temperature, filter with suction, redissolve the filter cake with 100mL acetone, and filter with suction. The organic phases were combined, and the organic solvent was removed under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (gradient elution, developer: first use ethyl acetate to remove the unreacted p-toluenesulfonyl substituted tetraethylene glycol, and then use anhydrous methanol / ethyl acetate=1 / 25 , volume ratio) to obtain long-chain alkoxy-substituted p-bromodiphenyl (monomer I) 2.1g, yield: 33.8%. 1 HNMR (400MHz, CDCl 3 ,ppm):7.15(s,2H),4.12(t,4H),3.85(t,4H),3.75(t,4H),3.69(m,...
Embodiment 2
[0029] 1) The preparation of monomer (I) and monomer (II) is the same as in Example 1.
[0030] 2) Under nitrogen protection, in a 50mL four-necked flask equipped with mechanical stirring, add 0.31g (0.5mmol) of long-chain alkoxy-substituted p-bromodiphenyl (monomer I), 0.426g (0.6mmol) of alkoxy group-modified fluorene boronate (monomer II), and 15 mL of toluene. Add 5 mL of aqueous solution containing 0.552 g (4.0 mmol) of potassium carbonate into the above solution, and then add 0.1 g of Pd(dppf)Cl 2 Add it in, and raise the temperature to 110°C under stirring, and react for 48 hours. After the reaction, the solution was concentrated under reduced pressure to 2 mL, and the organic phase was washed twice with 30 mL of chloroform as a solvent and an equal volume of water. The organic phase was filtered, dried over anhydrous sodium sulfate, and the crude product was spin-off from the solvent. The crude product was treated with a dialysis bag with a molecular weight of 3500 to ...
Embodiment 3
[0032] 1) The preparation of monomer (I) and monomer (II) is the same as in Example 1.
[0033] 2) Under nitrogen protection, in a 50mL four-necked flask equipped with mechanical stirring, add 0.31g (0.5mmol) of long-chain alkoxy-substituted p-bromodiphenyl (monomer I), 0.426g (0.6mmol) of alkoxy group-modified fluorene boronate (monomer II), and 15 mL of toluene. Add 0.276g (2.0mmol) of potassium carbonate in 7.5mL of aqueous solution to the above solution, and then add 0.1g of Pd(dppf)Cl 2 Add it in, and raise the temperature to 110°C under stirring, and react for 48 hours. After the reaction, the solution was concentrated under reduced pressure to 2 mL, and the organic phase was washed twice with 30 mL of chloroform as a solvent and an equal volume of water. The organic phase was filtered, dried over anhydrous sodium sulfate, and the crude product was spin-off from the solvent. The crude product was treated with a dialysis bag with a molecular weight of 3500 to obtain 0.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com