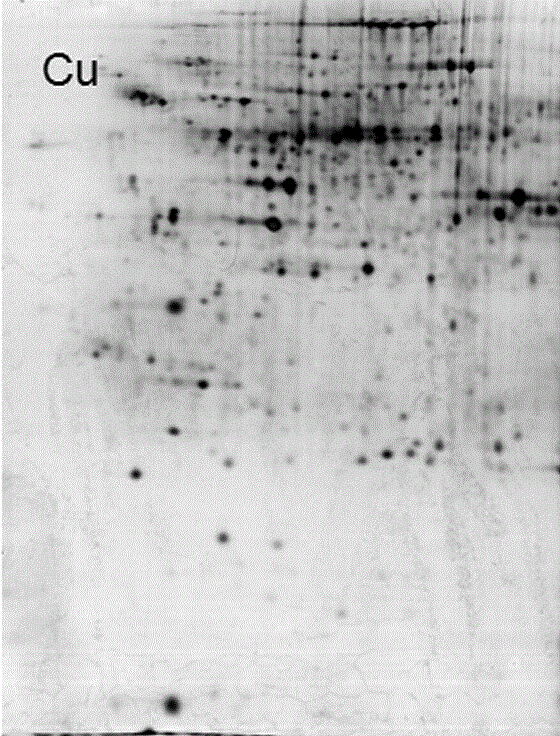
Method for separating specific metal-binding proteins by using protein denaturation
A metal-binding protein and protein denaturation technology, applied in the field of protein chemistry, can solve the problems of incomplete separation, inability to separate metal-binding proteins, and difficulty in separating metal-binding proteins, so as to avoid aggregation and precipitation, stabilize complexes, and improve the yield efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Extraction of plant protein
[0035] Use 100μmolL -1 For the germinated rice seed embryos treated with copper sulfate, fresh rice seed embryos were taken after 5 days of copper treatment, fully ground with liquid nitrogen, then added 4 times the volume of protein extract solution and mixed well, then extracted at 4°C for 30 minutes, and extracted at 4°C Centrifuge at 15,000×g for 30 min at ℃, and collect the supernatant. The denatured protein was quantified by a modified Bradford method (Ramagli, 1999) with chicken ovalbumin as the standard protein. The composition of the protein extract is as follows: every liter of protein solution contains 50mmol / L sodium phosphate, pH7.8, 300mmol / L sodium chloride, 0.1-1% TritonX-100 by mass percentage, 8mol / L urea, The remainder is water.
[0036] 2. Cu-NTA agarose affinity chromatography process
[0037] (1) Packing: Add 2ml of Ni-NTA agarose (GE Healthcare) into the glass tube along the tube wall, and push the piston to a ...
Embodiment 2
[0046] A method for separating a specific metal-binding protein by protein denaturation, comprising the following steps:
[0047] (1) Take biological tissues or cells and grind them fully with liquid nitrogen, add protein extract, mix well, and centrifuge at low temperature to obtain denatured protein solution; The solid-to-liquid ratio of the extract is: 1:4; the composition of the protein extract is: every liter of protein solution contains 50mmol / L sodium phosphate, pH7.8, 300mmol / L sodium chloride, and the mass percentage is 0.1- 1% TritonX-100, 8mol / L urea, the balance is water;
[0048] (2) Ni in the purchased Ni-NTA agarose (GE Healthcare) 2+ Replaced with Cu 2+ Load it into a chromatography column; inject the denatured protein solution obtained in the above step (1) into Cu-NTA agarose at a flow rate of 0.25ml / min, and incubate at 37°C for 15-40min to fully bind the denatured protein; Then, under low temperature conditions, slowly inject eluent 1 at a flow rate of 0...
Embodiment 3
[0057] A method for separating a specific metal-binding protein by protein denaturation, comprising the following steps:
[0058] (1) Take biological tissues or cells and grind them fully with liquid nitrogen, add protein extract, mix well, and centrifuge at low temperature to obtain denatured protein solution; the amount of protein extract added is, after grinding, biological tissue or cells and protein The solid-to-liquid ratio of the extract is: 1:4; the composition of the protein extract is: every liter of protein solution contains 50mmol / L sodium phosphate, pH7.8, 300mmol / L sodium chloride, and the mass percentage is 0.2% TritonX-100, 8mol / L urea, the balance is water;
[0059] (2) Replace Ni in the purchased Ni-NTA agarose (GE Healthcare) with Cu and load it into the chromatographic column; inject the denatured protein solution obtained in the above step (1) into Cu-NTA at a flow rate of 0.25ml / min Agarose, incubate at 37°C for 15-40min to fully bind the denatured prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com