Method for preparing (R)-3-quinuclidinol
A technology for quinuclidinol and quinuclidinone is applied in the field of preparing -3-quinuclidinol, and can solve the problems of low total yield, many splitting steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
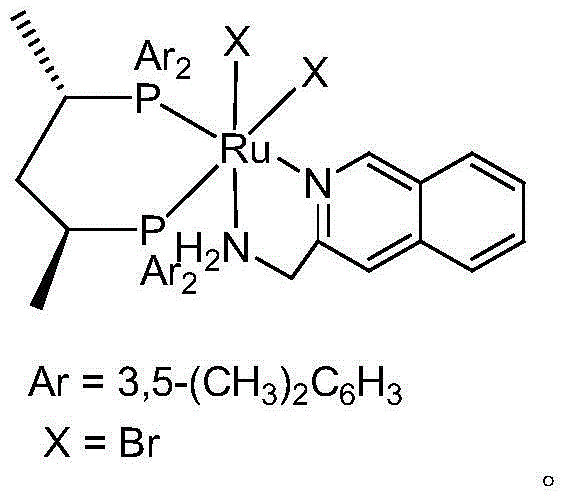
[0043] Preparation of Chiral Catalyst (S,S)xylskewphosRuBr 2 QUIMA, hereinafter referred to as QUIMA catalyst.
[0044] Put 1.5g (s,s)-xylskewphos and 0.895g Ru[η3-CH2(CH3)CH2]2(cod) into 100ml schlenk, replace the air with argon, add 70ml n-hexane, and react at 70°C for 2h. After the reaction was completed, it was filtered, and the obtained filtrate was sucked dry to obtain a solid.
[0045] 60 ml of acetone was added to the above solid, and after the solid was completely dissolved, 0.6 ml of 48% HBr aqueous solution was slowly added dropwise. After half an hour of reaction at room temperature, the system solvent was drained.
[0046] Under argon, 0.35 g of QUIMA was added to the above schlenk. Add 60ml of DMF and stir at room temperature for 24h. After the reaction was completed, the DMF was sucked dry, 6ml of dichloromethane and 60ml of n-hexane were added, stirred, and a solid was precipitated, filtered, and the solid was washed with 20ml of n-hexane. The solid was su...
Embodiment 2
[0048] Add 74kg of water into the 200L reactor, then add 9kg of solid sodium hydroxide, stir and cool down to 10°C. 18kg3-quinine hydrochloride was added to the reactor, and the reaction was stirred. The reaction system was extracted three times with 288kg of dichloromethane (96kg×3). The organic phases are then combined. Heating and vacuum distillation gave a white solid, which was then vented with nitrogen. Under the protection of nitrogen, 34 kg of ethanol was added into the reactor, and the solution in the reactor was transferred to the hydrogenation autoclave. Argon was bubbled through for 1.5 hours. Then, under the protection of argon, 0.2 kg of potassium tert-butoxide and 1.3 g of QUIMA catalyst were successively added. Fill the autoclave with hydrogen to 0.8Mpa, replace it with hydrogen 4 times, then add hydrogen to 3.0MPa, the reaction temperature is 30°C, and stir the reaction. After the reaction is completed, slowly vent the pressure in the reactor to normal pr...
Embodiment 3
[0050]Add 74kg of water into a 200L reactor, and then add 9.8kg of solid potassium tert-butoxide, stir and cool down to 10°C. 18kg3-quinine hydrochloride was added to the reactor, and the reaction was stirred. The reaction system was extracted three times with 288kg of dichloromethane (96kg×3). The organic phases are then combined. Heating and vacuum distillation gave a white solid, which was then vented with nitrogen. Under the protection of nitrogen, 34 kg of ethanol was added into the reactor, and the solution in the reactor was transferred to the hydrogenation autoclave. Argon was bubbled through for 1.5 hours. Then, under the protection of argon, 0.2 kg of potassium tert-butoxide and 1.3 g of QUIMA catalyst were sequentially added. Fill the autoclave with hydrogen to 0.8Mpa, replace it with hydrogen 4 times, then add hydrogen to 3.0MPa, the reaction temperature is 50°C, and stir the reaction. After the reaction is completed, slowly vent the pressure in the reactor to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com