A kind of synthetic method of milbexime
A synthesis method, the technology of milbexime, which is applied in the field of synthesis of semi-synthetic macrolide anthelmintic drug milbexime, can solve the problems of chromium trioxide toxicity, heavy metal pollution, and many side reactions, and achieve low cost, Less side effects and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
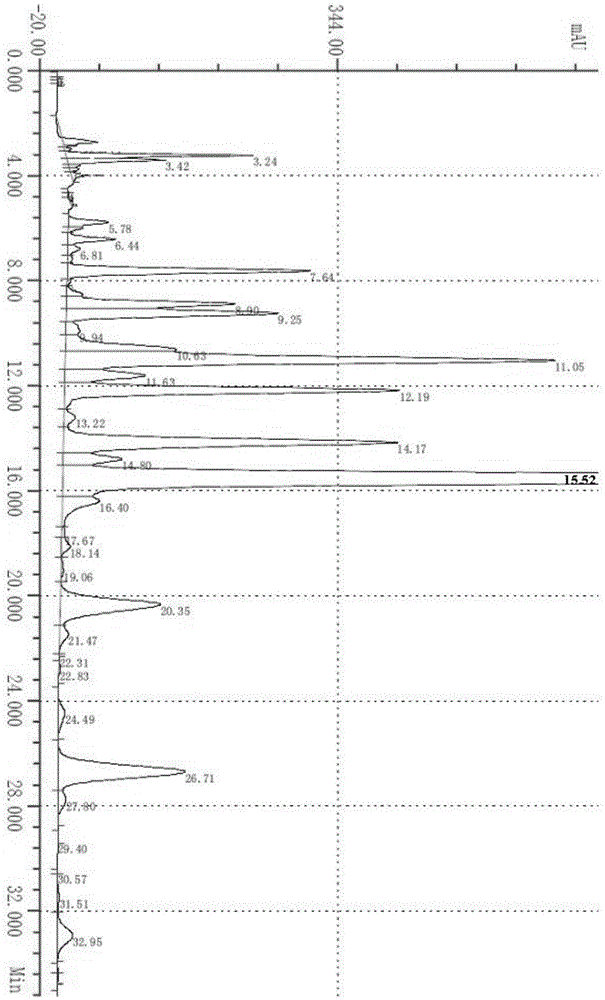
[0023] Contain 5Kg milbemycin (C 31 h 43 o 7 or C 32 h 45 o 7 ) of the raw material 24.7Kg is placed in the reactor, and the spectrogram obtained by the milbemycin liquid chromatography detection used in the present embodiment is as follows figure 1 as shown, figure 1 Among them, the peak at 11.11min is Milbemycin A 3 , the peak at 13.92min is Milbemycin A 4 .
[0024] Add 300g of 2,2,6,6-tetramethylpiperidine-N-oxyl radical into the reaction kettle, add 100L of dichloromethane to dissolve, set the temperature of the refrigeration equipment to 5°C, turn on the refrigeration, and turn on the stirring. Weigh 200g of sodium bromide, dissolve it in 1000ml of deionized water and add it to the reaction solution;
[0025] (2), take by weighing 3.57Kg sodium bicarbonate and 11.76Kg sodium carbonate, add 100L water to dissolve, add 81Kg20% sodium hypochlorite solution, stir well, adjust pH to 10 ± 0.5;
[0026] (3), the oxidant solution is divided into 5 batches and added dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com