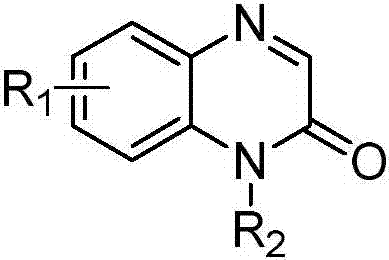
A kind of synthetic method of quinoxaline-2(1h)-ketone C-3 phosphonic acid compound
A synthesis method and quinoxaline technology are applied in the field of synthesis of quinoxaline-2-one C-3 phosphonic acid compounds, and can solve the problems of harsh reaction conditions, complicated reaction steps, narrow substrate range and the like. Easy to handle, green post-processing, wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of Dimethyl(3-oxo-3,4-dihydroquinoxalin-2yl)phosphonate
[0023] Put 0.2mmol of quinoxalin-2(1H)-one, 0.6mmol of dimethyl phosphate, 0.6mmol of potassium persulfate, and 2.0mL of acetonitrile into a 10mL reaction tube, place in an oil bath at 100°C, and Under the reaction 8h. Stop the reaction and cool to room temperature. The reaction solution was spin-dried with a rotary evaporator, and separated by column chromatography to obtain 46.7 mg of the target product with a yield of 92%. The compound is characterized as follows: 1 H NMR (400MHz, d 6 -DMSO) δ12.79(s, 1H), 7.89(d, J=8.0Hz, 1H), 7.69-7.63(m, 1H), 7.40-7.33(m, 2H), 3.87(d, J=11.1Hz , 6H). 13 C NMR (100MHz, d 6 -DMSO) δ154.5 (d, J C-P =31.4Hz), 153.8(d, J C-P =223.8Hz), 133.5(s), 133.2(d, J C-P =2.9Hz), 132.0(d, J C-P =25.3Hz), 130.3(s), 124.3(s), 116.3(s), 54.5(d, J C-P =6.3Hz). 31 P NMR (162MHz, d 6 -DMSO)δ9.4(s).HRMS m / z(ESI)calculated for C 10 h 11 N 2 o 4 P(M+H) + 255.0535, foun...
Embodiment 2
[0025] Preparation of Diethyl(3-oxo-3,4-dihydroquinoxalin-2yl)phosphonate
[0026] Put 0.2mmol of quinoxalin-2(1H)-one, 0.6mmol of diethyl phosphate, 0.6mmol of potassium persulfate, and 2.0mL of acetonitrile into a 10mL reaction tube, place in an oil bath at 100°C, and Under the reaction 8h. Stop the reaction and cool to room temperature. The reaction solution was spin-dried with a rotary evaporator, and separated by column chromatography to obtain 47.9 mg of the target product with a yield of 85%. The compound is characterized as follows: 1 H NMR (400MHz, d6-DMSO) δ 12.78 (s, 1H), 7.88 (d, J = 7.9Hz, 1H), 7.70-7.63 (m, 1H), 7.42-7.31 (m, 2H), 4.26 ( dq, J1=14.2Hz, J2=7.1Hz, 4H), 1.32(t, J=7.0Hz, 6H). 13 C NMR (100MHz, d6-DMSO) δ154.4(d, JC-P=31.4Hz), 154.2(d, JC-P=222.9Hz), 133.4(s), 133.2(d, JC-P=2.6Hz ), 131.9(d, JC-P=25.2Hz), 130.3(s), 124.3(s), 116.3(s), 63.8(d, JC-P=6.2Hz), 16.8(d, JC-P=6.1 Hz). 31 P NMR (162MHz, d6-DMSO) δ7.0(s). HRMS m / z (ESI) calculated for C1...
Embodiment 3
[0028] Preparation of Diisopropyl(3-oxo-3,4-dihydroquinoxalin-2yl)phosphonate
[0029] Add 0.2mmol of quinoxalin-2(1H)-one, 0.6mmol of diisopropyl phosphate, 0.6mmol of potassium persulfate, and 2.0mL of acetonitrile into a 10mL reaction tube, place in an oil bath at 100°C, and air Under the condition of reaction 8h. Stop the reaction and cool to room temperature. The reaction solution was spin-dried with a rotary evaporator, and separated by column chromatography to obtain 41.8 mg of the target product with a yield of 67%. The compound is characterized as follows: 1 H NMR (400MHz, d 6 -DMSO) δ12.74 (s, 1H), 7.85 (d, J=7.9Hz, 1H), 7.69-7.63 (m, 1H), 7.37 (dd, J 1 =14.1,J 2 =7.4Hz, 2H), 4.82(dq, J 1 =12.4,J 2 =6.2Hz, 2H), 1.35(dd, J=6.0Hz, J 2 =4.7Hz, 12H). 13 C NMR (100MHz, d 6 -DMSO) δ154.6 (d, J C-P =223.5Hz), 154.3(d, J C-P = 31.4Hz), 133.2(s), 133.1(d, J C-P =2.6Hz), 130.2(s), 124.3(s), 116.2(s), 72.23(d, J C-P =6.2Hz), 24.57(d, J C-P =3.2Hz), 23.91(d, J C-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com