Monoclonal antibody purification process
A technology of monoclonal antibodies and process methods, applied in chemical instruments and methods, peptide preparation methods, organic chemistry, etc., can solve problems such as low resolution and poor separation effect, achieve improved purity, improved main peak purity, and improved antibody The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Herceptin monoclonal antibody purification
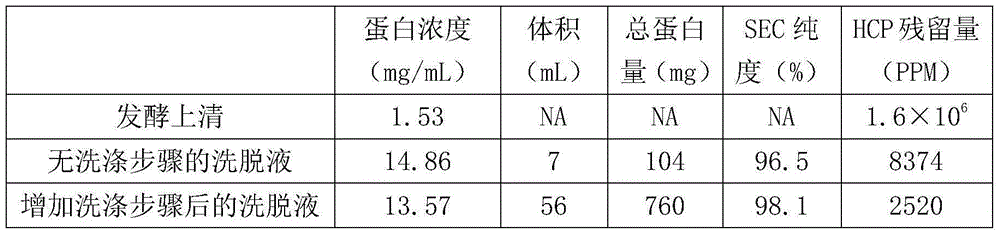
[0024] 1. Protein A affinity chromatography
[0025] Step 1, wash the chromatography column with 3 times the column volume of water for injection, then sterilize the chromatography column with a mixed solution of 3 times the column volume of NaOH (50mM) and NaCl (1M), and then use 3 times the column volume of water for injection Rinse the column.
[0026] Step 2, equilibrate the chromatography column with 5 times the column volume of buffer solution (20mM disodium hydrogen phosphate-sodium dihydrogen phosphate + 150mM NaCl, pH7.4).
[0027] Step 3, sample loading, let the sample stay on the column for 4 minutes.
[0028] Step 4, wash with 20mM disodium hydrogen phosphate-sodium dihydrogen phosphate+150mM NaCl, pH7.4 to A280 baseline, and then use 5 times column volume buffer (20mM citric acid-sodium citrate+1M NaCl, pH5. 5) Wash the chromatographic column, and then wash the chromatographic column with 3 times ...
Embodiment 3
[0062] Embodiment 3 Rituximab monoclonal antibody purification
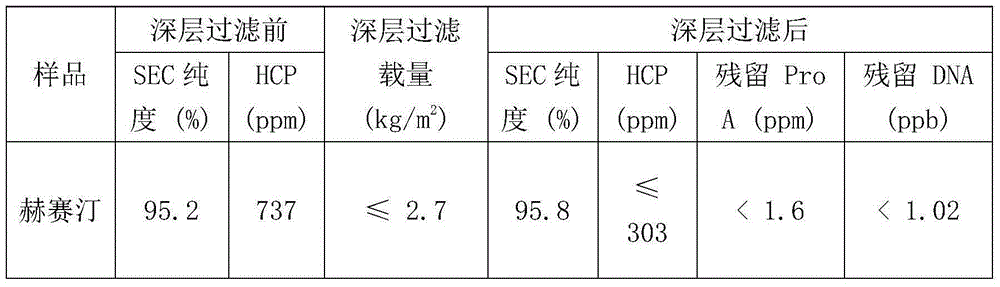
[0063] The purification process is the same as in Example 1, and the HCP and DNA residues and SEC purity of the affinity chromatography eluate after one-step deep filtration are shown in Table 6:
[0064] Table 6 HCP and DNA residues and SEC purity of affinity chromatography eluate after deep filtration
[0065]
[0066] It can be seen from Table 6 that after one-step deep filtration of the affinity chromatography eluate, the HCP residual content was significantly reduced, from 794ppm to 127ppm, and the DNA residue also fully complied with FDA requirements.
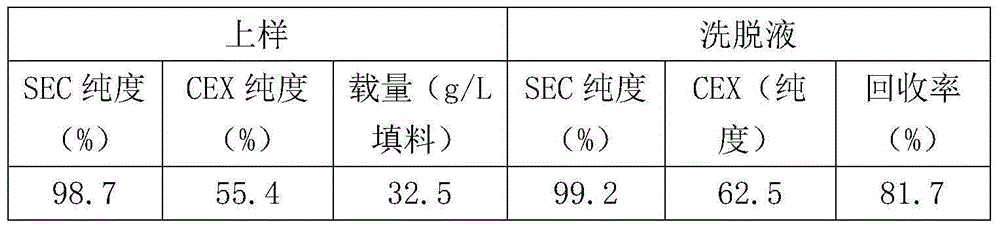
[0067] The purity and residue indicators of the final product are shown in Table 7:
[0068] Table 7 adopts the purity and residual index of the product prepared by process purification of the present invention
[0069]
[0070] It can be seen from Table 7 that the purity and residual indicators of the rituximab product purified and prepared by the p...
Embodiment 4
[0071] Example 4 MabThera Monoclonal Antibody Purification
[0072] The processing method of purification is the same as embodiment 1, and the purity and residual index of final product are referring to table 8:
[0073] Table 8 adopts the purity and residual index of the product prepared by process purification of the present invention
[0074]
[0075] It can be seen from Table 8 that the purity and residual indicators of the MabThera monoclonal antibody product purified and prepared by the process of the present invention also meet the requirements of sFDA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com