Dimethyl sulfone preparation method
A technology of dimethyl sulfone and dimethyl sulfide, which is applied in the field of preparation of dimethyl sulfone and achieves the effects of high conversion rate and cost saving of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
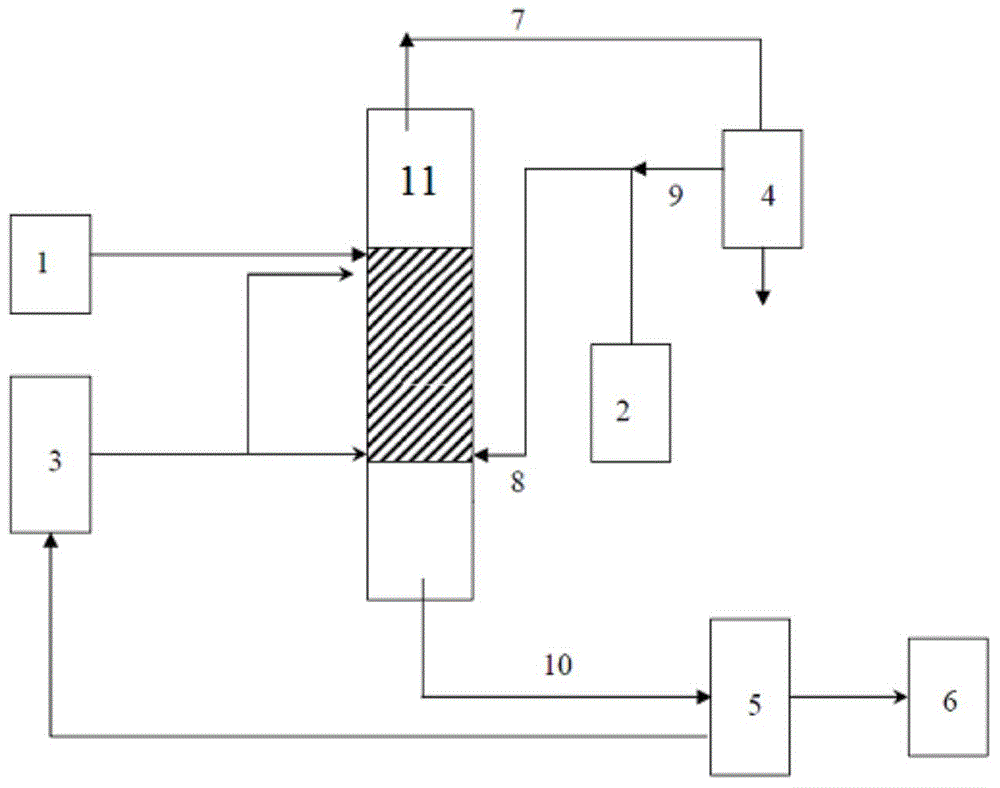
[0011] The invention provides a method for the preparation of dimethyl sulfone, the method comprising, in a catalytic distillation reactor having at least one reaction zone, contacting a liquid mixture with a catalyst in the reaction zone to obtain dimethyl sulfone containing A stream, the liquid mixture contains dimethyl sulfide and at least one oxidizing agent, and the catalyst contains at least one titanium silicate molecular sieve.
[0012] The present invention has no particular limitation on the method of feeding the dimethyl sulfide and the oxidizing agent into the reaction zone. Preferably, the oxidizing agent is sent into the reaction zone from the first feed port, the dimethyl sulfide is sent into the reaction zone from the second feed port, and the first feed port is fed into the reaction zone. The number of theoretical plates at the bottom of the reaction zone is T 1 , the number of theoretical plates from the second feed port to the bottom of the reaction zone is...
Embodiment 8 and 11
[0060] Embodiment 8 and 11 adopt the following method to measure the activity of catalyst:
[0061] Catalyst, 36% by weight of ammonia water (as NH 3 meter), 30% by weight of hydrogen peroxide (as H 2 o 2 ), tert-butanol and cyclohexanone by mass ratio = 1:7.5:10:7.5:10 and then stirred and reacted at 80°C under atmospheric pressure for 2h, filtered the reactant, and analyzed the obtained liquid phase by gas chromatography The composition of the catalyst is analyzed, and the conversion rate of cyclohexanone is calculated by the following formula and used as the activity of the catalyst,
[0062] The conversion rate (%) of cyclohexanone=[(the molar amount of cyclohexanone added−the molar amount of unreacted cyclohexanone) / the molar amount of cyclohexanone added]×100%.
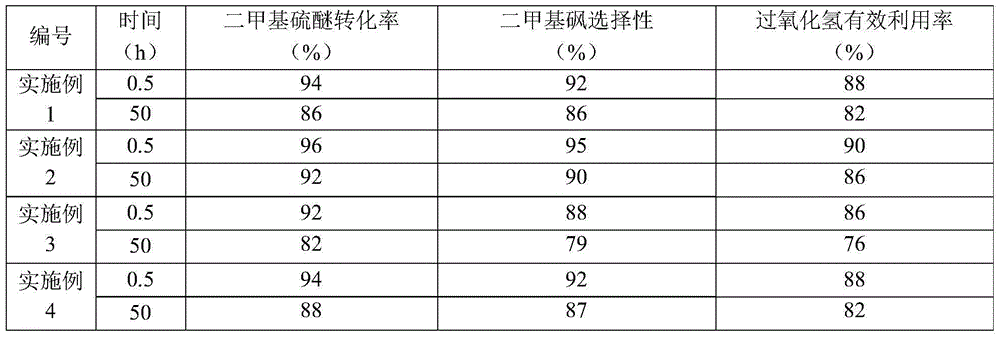
Embodiment 1
[0065] According to the mass ratio of dimethyl sulfide to hydrogen peroxide (concentration is 27.5% by weight) and acetone is 1:4.5:15 from the feed inlet of the reaction zone feed, wherein, dimethyl sulfide from the second feed Inlet feed, hydrogen peroxide and acetone are fed from the first feed inlet, the temperature in the middle of the reaction zone is 47±3°C, the pressure at the top of the reaction zone is 0.15±0.02MPa (absolute pressure), the heavy space-time of dimethyl sulfide speed is 2h -1 , the reflux ratio of the reaction zone is 5:1, the total number of theoretical plates in the reaction zone is 35, the theoretical plate number from the first feed port to the bottom of the reaction zone is 30, and the theoretical plate number from the second feed port to the bottom of the reaction zone is 30. The number of trays is 10, and catalyst (average particle diameter is the spherical catalyst of 5 μm) and θ ring filler (based on the total amount of catalyst and filler, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Radial length | aaaaa | aaaaa |
| Benzene adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com