Fluorine-containing structure methacrylate macromonomer without bisphenol-A structure and its preparation method and application
A technology of methacrylate and trimethylcyclohexyl isocyanate is applied in the preparation of carbamic acid derivatives, the preparation of organic compounds, dental preparations, etc., which can solve the problems affecting the growth and development of infants and the safety of dental restoration materials. , to achieve the effect of promoting photo-initiated polymerization, reducing oxygen inhibition, and high conversion of double bonds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
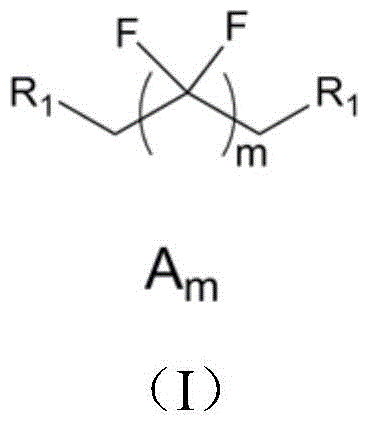
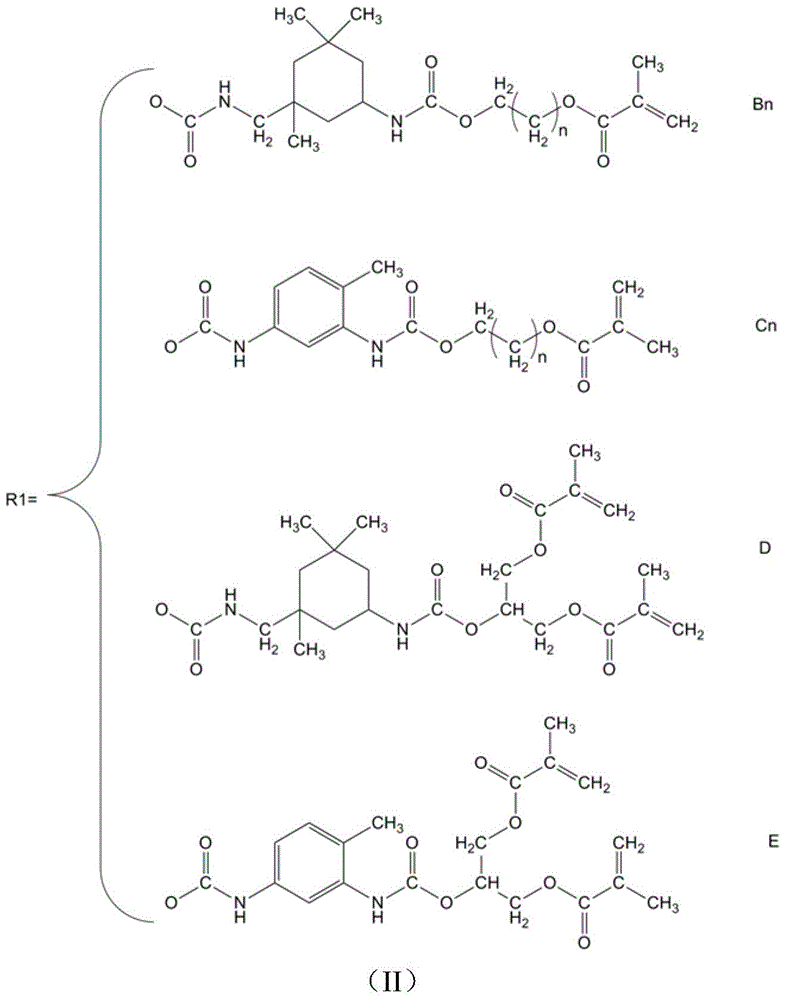
[0032] Preparation of DHOFH-IPDI-HEMA (A 4 B 1 ) large monomer:
[0033] The present embodiment DHOFH-IPDI-HEMA (A 4 B 1 ) The preparation method of macromer comprises the steps:
[0034] Add 6.67g of IPDI to a 250ml three-necked bottle equipped with a magnet, continuously add 3.93g of octafluoro-1,6-hexanediol (DHOFH) through a constant pressure dropping funnel under stirring, and rinse with 2g of tetrahydrofuran Constant pressure dropping funnel, react at 60°C for 0.5 hours, until the amount of -NCO in the diisocyanate system is consumed close to the theoretical value, adjust the temperature to 90°C in a water bath, and then add 3.91g to the reactor through the constant pressure dropping funnel Hydroxyethyl methacrylate (HEMA), 0.002g dibutyltin dilaurate and 0.11g hydroquinone were reacted, and the constant pressure dropping funnel was rinsed with tetrahydrofuran after adding the material, reacted for 2 hours, and then the reaction product was Purification treatment, y...
Embodiment 2
[0036] Preparation of DHOFH-IPDI-HEMA (A 4 B 1 ) large monomer
[0037] The present embodiment DHOFH-IPDI-HEMA (A 4 B 1 ) The preparation method of macromer comprises the steps:
[0038] Add 6.67g of IPDI to a 250ml three-necked bottle equipped with a magnet, and continuously add 3.93g of octafluoro-1,6-hexanediol (DHOFH) through a constant pressure dropping funnel under stirring, and use 2g of chloroform Rinse the constant pressure dropping funnel, react at 20°C for 10 hours, until the amount of NCO in the diisocyanate system is consumed close to the theoretical value, adjust the temperature to 40°C in a water bath, and then add to the reactor through the constant pressure dropping funnel 3.91g hydroxyethyl methacrylate (HEMA), 0.003g stannous octoate and 0.061g p-Hydroxyanisole are reacted, add the material and rinse the constant pressure dropping funnel with chloroform, react for 24 hours, then react the reaction product Purification treatment was carried out, and the ...
Embodiment 3
[0040] Preparation of DHOFH-TDI-HDMA (A 4 E) Large monomer
[0041] Add 17.4g of TDI into a 250ml three-neck bottle equipped with a magnet, and continuously add 13.1g of octafluoro-1,6-hexanediol (DHOFH) through a constant pressure dropping funnel under stirring, and rinse with 20mL of tetrahydrofuran Constant pressure dropping funnel, react at 60°C for 0.5 hours, until the amount of -NCO in the diisocyanate system is consumed close to the theoretical value, adjust the temperature to 45°C in a water bath, and then add 22.8g into the reactor through the constant pressure dropping funnel 2-Hydroxy-1,3-dimethylacryloyloxypropane (HDMA), 0.002g dibutyltin dilaurate and 0.11g hydroquinone were reacted, and the constant pressure dropping funnel was rinsed with tetrahydrofuran after adding the materials , reacted for 2 hours, and then purified the reaction product with a yield of 87%. FT-IR:ν(cm -1 ) 3335, 3068, 2950, 2923, 1720, 1636, 1608, 1585, 1242.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com