Dihydroagarofuran type sesquiterpene compound and preparing method and application thereof
A dihydro-agarwood and furan-type technology, applied in the field of preparation of antibacterial and insecticidal pesticides, can solve the problems of activity differences, no research reports on insecticidal and antibacterial activities, and non-existence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
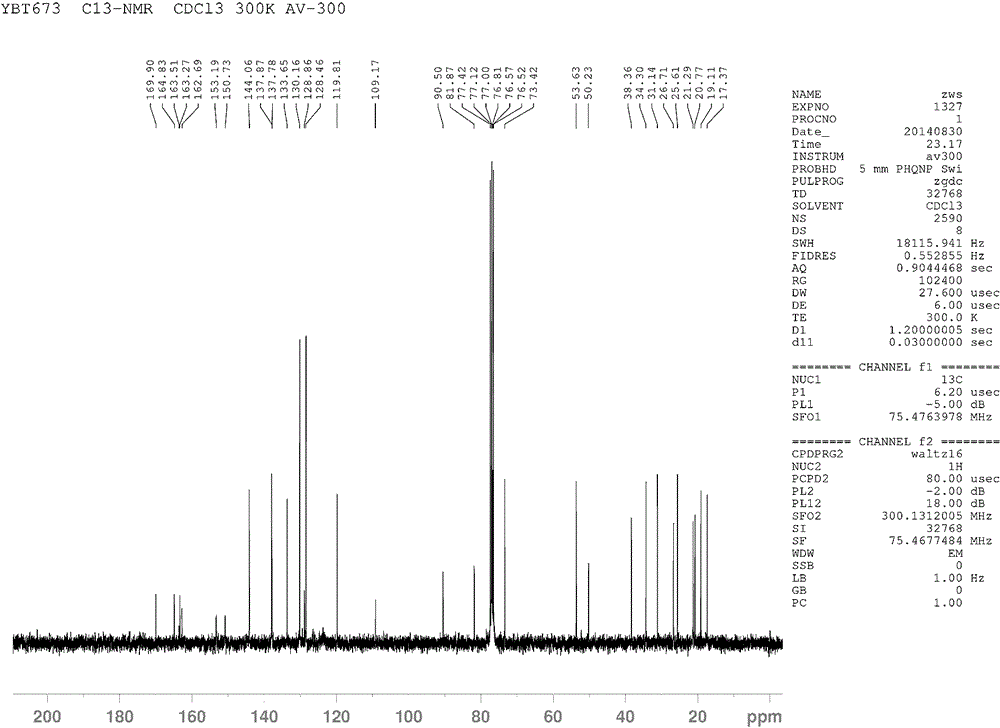
[0027] 5 kg of stems and leaves of the vine sesquiterpene were extracted with 90% ethanol at 60°C for 3 times, filtered and concentrated to obtain the extract, which was suspended in water and then extracted with ethyl acetate to obtain the ethyl acetate fraction. The ethyl acetate extract was chromatographed on D101 macroporous resin, and 50-80% alcohol eluted parts were collected, and then washed with C 18 Reverse-phase silica gel column, collect 70% of the elution fraction, and then use C 18 Prepare the liquid phase with a reversed-phase silica gel column, and collect 75% of the elution site to obtain compound 1 β -Acetyl-6 α -(5-carboxy-N-methyl-2-pyridinone)-8 β -Nicotinyl-9 α -Benzoyl- β - Dihydroagarfuran, the purity of which is greater than 98% as detected by HPLC-MS.
[0028] The structure of the compound can be resolved by combining the MS / MS data with the NMR spectrum. HR-ESI MS m / z673.2699[M+H] + , showing the molecular formula as C 37 h 40 N 2 o 10 , wi...
Embodiment 2
[0031] The preliminary toxicity determination of insecticides adopts the spray method.
[0032] (1) Preparation of medicines: The ethyl acetate part of the vine petals and the new sesquiterpene were prepared into an acetone solution of 1 mg / mL, then diluted 10 times with acetone, and set aside.
[0033] (2), test insect removal: take diamondback moth ( Plutella xylostella ) After the 3rd instar larvae were concentrated and mixed, they were brushed into the petri dish with a brush, and 10 larvae were treated each time, and repeated. Each treatment requires 15-30 test insects, and a blank control is required for each test. The temperature is generally 20-28°C, the relative humidity is 60-80%, and the ventilation is good.
[0034] (3) Spraying quality inspection: Use a 9cm white circular paper to check whether the size of the spray droplets and the spraying are uniform. If they do not meet the requirements, adjust the nozzle and position to make the spraying quality meet the ...
Embodiment 3
[0040] The contact activity of insecticides was determined by the drop method.
[0041] (1) Drug preparation: According to the preliminary toxicity test results, dilute the acetone mother liquor of 1mg / mL new sesquiterpene with acetone to 5 concentrations, each concentration is 5mL, and put it into a stoppered small test tube. And acetone was used as a control.
[0042] (2), Indoor tested pests: diamondback moth ( Plutella xylostella ),cabbage caterpillar( Pieris rapae L.), corn armyworm ( Mythimna separate a Walker), Spodoptera litura ( Prodenia litura F.), silver-leaved moth ( Plusia agnata Staudinger ), pea aphids ( Pea aphids ), rice stem borer ( Chilo suppressalis Walker), rice stem borer ( Tryporyza incertulas Walker), twenty-eight star ladybug ( Henosepilachna vigintioctopunctata ), the 3rd instar larvae with basically the same individual size were selected for the test.
[0043] (3) Spot treatment method: Pick neat 3rd instar larvae and put them...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com