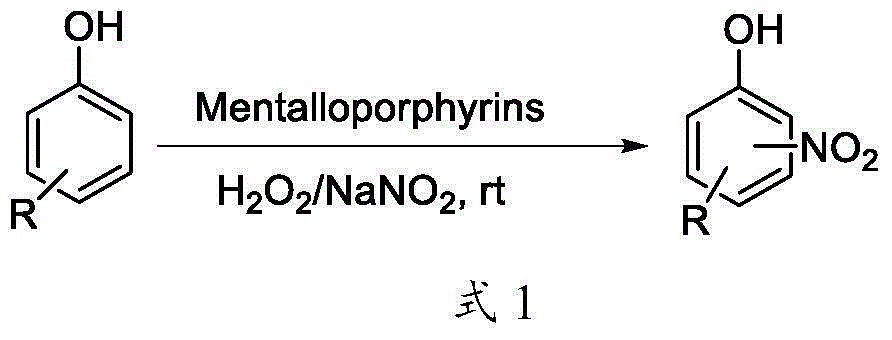Green nitrification method and application for phenolic compound
A phenolic compound, green technology, applied in the field of green nitration of phenolic compounds, can solve the problems of serious pollution, corroded equipment, severe conditions, etc., and achieve the effects of reducing environmental pollution, reducing corrosion, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] At room temperature, dissolve 1mmol of phenol in 40mL of pH=7 phosphate buffer, then add 50mmol of sodium nitrite, dissolve 3mmol of hydrogen peroxide in 10mL of phosphate buffer, drop into the reaction solution within 15min, Next, 0.05 g of water-soluble tetrakis(p-carboxyphenyl)iron porphyrin was added to start the reaction, and the reaction was stirred for 80 min. Extract with ethyl acetate (2×80mL), concentrate under reduced pressure, and separate by column chromatography (fill the column with silica gel, use petroleum ether and acetone as the eluent, and the volume ratio of petroleum ether and acetone is 3:1) to obtain the target product O-nitrophenol, yield 23.7% ( 1 H NMR (600MHz, DMSO-d 6 )δ10.92(s,1H),7.88(s,1H),7.53(s,1H),7.13(s,1H),6.98(s,1H); 13 C NMR (150MHz, DMSO-d 6 )δ152.52(s), 137.31(s), 135.71(s), 125.59(s), 119.77(s), 119.54(s); HR-ESI-MS m / z: Calcd for C 6 h 4 NO 3 {[M-H] -}:138.0197, found 138.0195), and p-nitrophenol, yield 21.5% ( 1 H NMR ...
Embodiment 2
[0041] At room temperature, dissolve 1mmol of phenol in 40mL of pH=7 phosphate buffer, then add 60mmol of sodium nitrite, dissolve 3mmol of hydrogen peroxide in 10mL of phosphate buffer, drop into the reaction solution within 15min, Next, 0.06 g of water-soluble tetrakis(p-sodium sulfonate phenyl)iron porphyrin was added to start the reaction, and the reaction was stirred for 80 min. Extract with ethyl acetate (2×80mL), concentrate under reduced pressure, and separate by column chromatography (fill the column with silica gel, use petroleum ether and acetone as the eluent, and the volume ratio of petroleum ether and acetone is 3:1 to obtain the target product o-nitrophenol, yield 27.3%, and p-nitrophenol, yield 28.8%.
Embodiment 3
[0043] At room temperature, dissolve 1mmol of phenol in 40mL of pH=7 phosphate buffer, then add 60mmol of sodium nitrite, dissolve 4mmol of hydrogen peroxide in 10mL of phosphate buffer, drop into the reaction solution within 15min, Next, 0.06 g of water-soluble tetrakis(p-sodium sulfonate phenyl)iron porphyrin was added to start the reaction, and the reaction was stirred for 80 min. Extract with ethyl acetate (2×80mL), concentrate under reduced pressure, and separate by column chromatography (fill the column with silica gel, use petroleum ether and acetone as the eluent, and the volume ratio of petroleum ether and acetone is 3:1 to obtain the target product o-nitrophenol, yield 26.9%, and p-nitrophenol, yield 27.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com