Sodium prasterone sulfate sustained-release tablet and preparation process thereof
A technology of prasterone sulfate sodium and sustained-release tablets, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., and can solve problems that need to be deepened
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] According to the second aspect of the present invention, the present invention also proposes a method for preparing the aforementioned prasterone sulfate sodium sustained-release tablet. The preparation method of the prasterone sulfate sodium sustained-release tablet according to specific embodiments of the present invention comprises:
[0053] (1) The prasterone sulfate sodium, skeleton material, filler and lubricant are pulverized respectively.
[0054] According to a specific embodiment of the present invention, in this step, the sodium prasterone sulfate, the skeleton material, the filler and the lubricant are pulverized to a particle size of 60-200 mesh. Thus, the solubility and bioavailability of Prasterone Sulfate Sodium can be effectively improved, and if the particle size is too high or too low, the solubility and bioavailability of the obtained Prasterone Sulfate Sodium Sustained-release Tablets are not ideal. In a preferred embodiment of the present inventio...
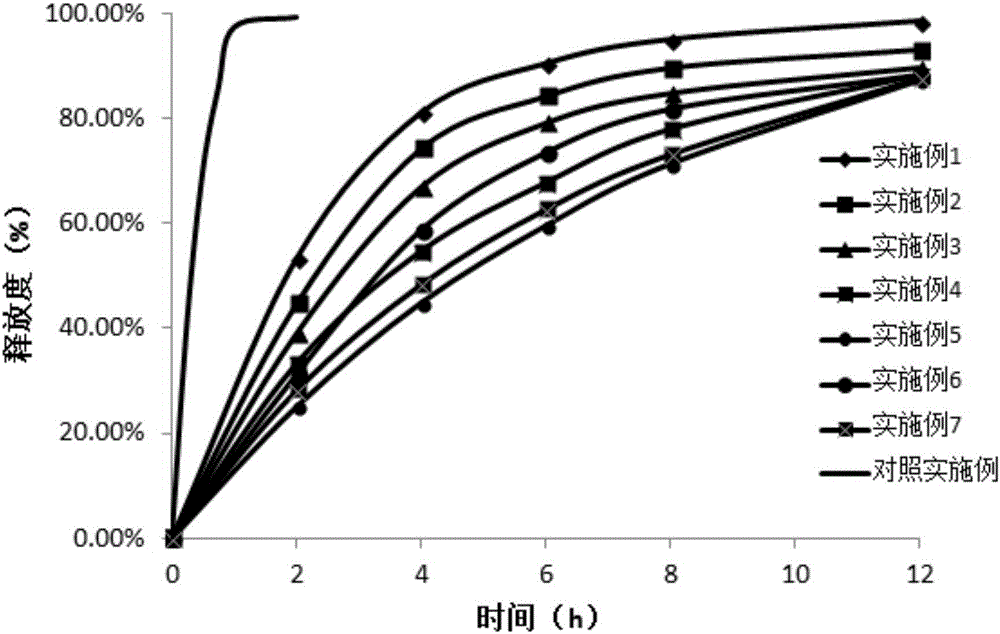
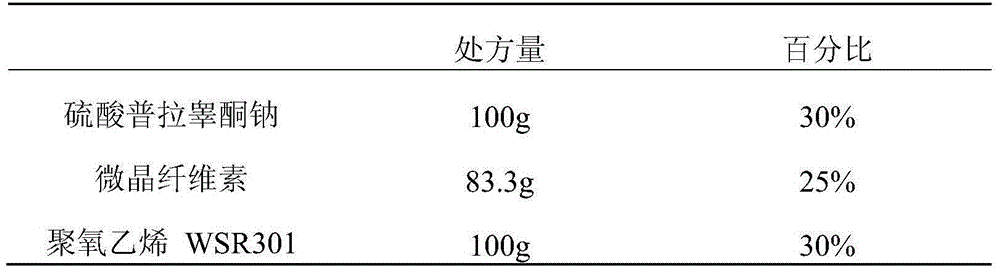
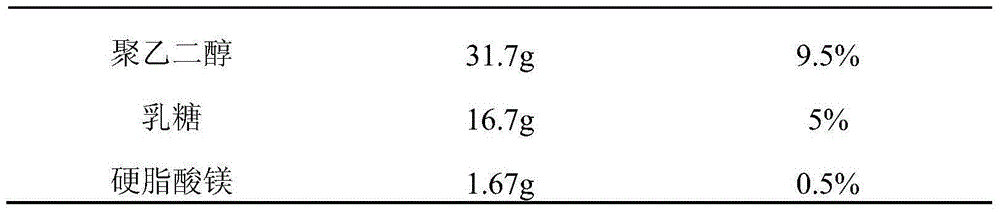
Embodiment 1
[0065]
[0066]
[0067] Preparation:
[0068] (1) Crushing the raw and auxiliary materials respectively and passing through a 60-mesh sieve for subsequent use;
[0069] (2) Premix the prescribed amount of prasterone sulfate sodium and polyoxyethylene WSR301 for 3 minutes, then add microcrystalline cellulose, polyethylene glycol and lactose and mix for 2 minutes.
[0070] (3) Add an appropriate amount of magnesium stearate, mix well, and compress into tablets, and the pressure is controlled at 8-9 kg during tablet compression.
Embodiment 2
[0072]
[0073] Preparation:
[0074] (1) Crushing the raw and auxiliary materials respectively and passing through an 80-mesh sieve for subsequent use;
[0075] (2) Weigh the prescribed amount of prasterone sulfate sodium and polyoxyethylene WSR303 and pre-mix for 4 minutes, then add microcrystalline cellulose, polyethylene glycol and lactose and mix for 2 minutes.
[0076] (3) Add an appropriate amount of magnesium stearate, mix evenly, and compress into tablets, and the pressure is controlled at 9-10 kg during tablet compression.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com