Synthesis method of 2,8-diaryl(amino) Troger's base derivatives
A technology of amino chaogle base and chaogle base, which is applied in the field of synthesis of chaogle base derivatives, can solve the problem of lack of Kang liver cancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
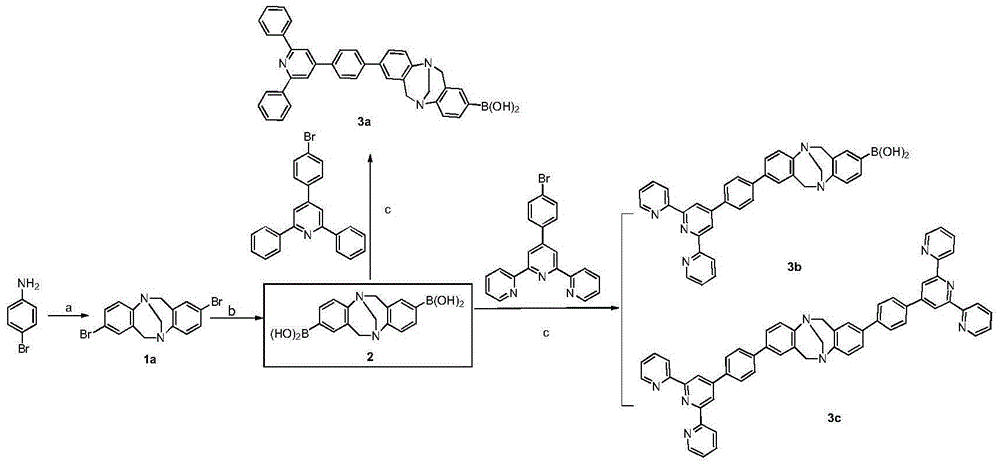
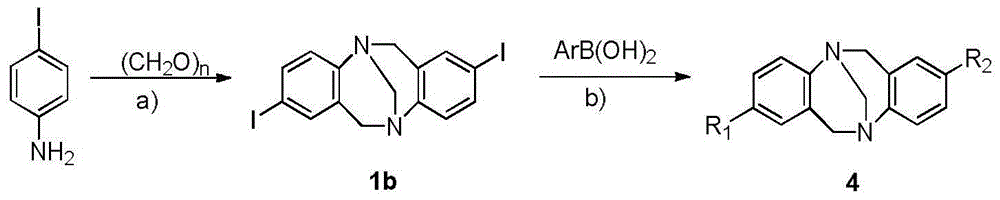
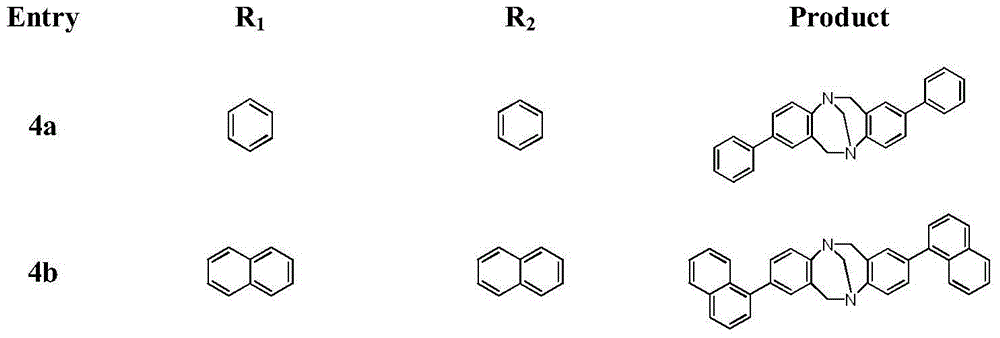
[0048] Embodiment 1: 2,8-diaryl (amino) Chaoger base derivatives of the present invention include: 2(8)-triarylpyridine (tripyridine) Chaoger base (TB) derivatives, 2,8 - diaryl Chaoger base (TB) derivatives and 2-aryl-8-amino Chaoger base (TB) derivatives;
[0049] 1. Synthesis method of 2(8)-triarylpyridine (tripyridine) Chaoger base derivative, substance of "2(,8)-triarylpyridine (tripyridine) Chaoger base (TB) derivative" The structure is as follows:
[0050]
[0051] (a) TFA, -15℃-0℃, 6d; (b) n-BuLi, trimethyl borate, -78℃-r.t.; (c) Pd(PPh 3 ) 4 , K 2 CO 3 ,toluene,110℃(THF,70℃)
[0052] The method comprises the steps of:
[0053] 1) Synthesis of compound 1a, i.e. 2,8-dibromochauger base:
[0054] Add 60mmol p-bromoaniline and 120mmol paraformaldehyde into a 250mL dry three-necked round-bottom flask at -15°C, slowly drop 120mL trifluoroacetic acid (TFA) into it with a constant pressure funnel under stirring, the solution turns maroon, after the addition is compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com