Synthesis of 2,8-diaryl(amino) Chaoger base derivatives and their anti-hepg2 activity in human liver cancer
A synthetic method, technology of chaogle base, applied in the synthesis of 2,8-diaryl chaogle base derivatives, in the field of anti-human liver cancer HepG2 activity, can solve the problem of lack of Kang liver cancer drugs, and achieve green reaction , High practical application value, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
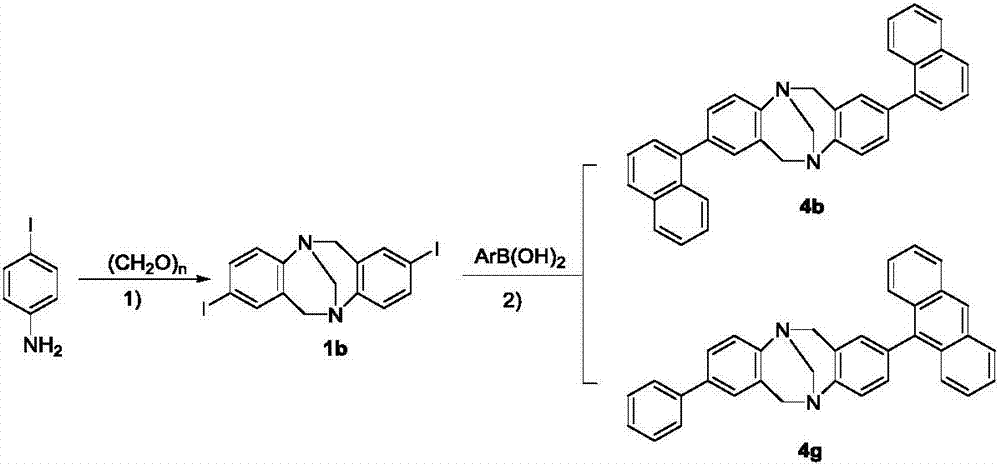
[0046] Embodiment 1: 2,8-diaryl (amino) Chaoger base derivatives of the present invention include: 2,8-triarylpyridine (tripyridine) Chaoger base (TB) derivatives, 2,8- Diaryl Chaoger base (TB) derivatives and 2-aryl-8-amino Chaoger base (TB) derivatives;
[0047] 1. The synthetic method of 2,8-triarylpyridine (tripyridine) Chaoger base derivative, the substance structure of "2,8-triarylpyridine (tripyridine) Chaoger base (TB) derivative" is as follows:
[0048]
[0049] The method comprises the steps of:
[0050]
[0051] (a) trifluoroacetic acid, -15°C-0°C, 6d; (b) n-butyl lithium, trimethyl borate, -78°C-r.t.; (c) tetrakistriphenylphosphine palladium, potassium carbonate, toluene, 110°C;
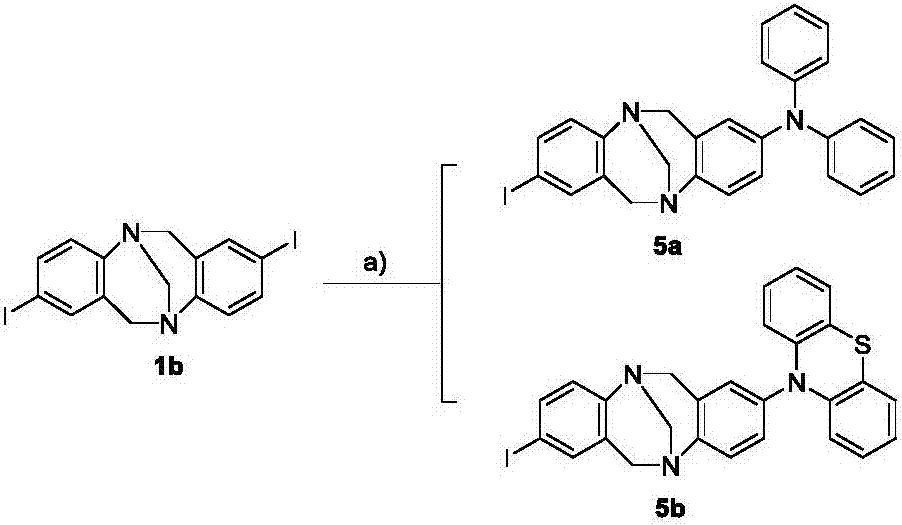
[0052] 1) Synthesis of compound 1a, i.e. 2,8-dibromochauger base:
[0053] Add 60mmol of p-bromoaniline and 120mmol of paraformaldehyde into a 250mL dry three-neck round bottom flask at -15°C, and slowly drop 120mL of trifluoroacetic acid into it with a constant pressure funnel u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com