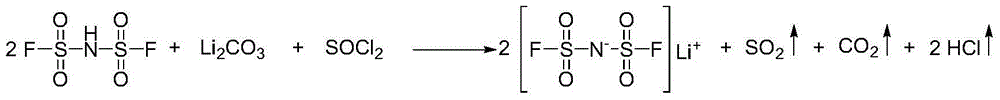Preparation method for difluorosulfimide lithium salt
A technology of bisfluorosulfonimide lithium salt and bisfluorosulfonimide is applied in the field of preparation of bisfluorosulfonimide lithium salt, which can solve the problem that LiF and LiFSI are not easily separated, the purity of LiFSI cannot be guaranteed, and it is not suitable for Industrial production and other problems, to achieve the effect of low cost, convenient recycling and application, and ensuring quality and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
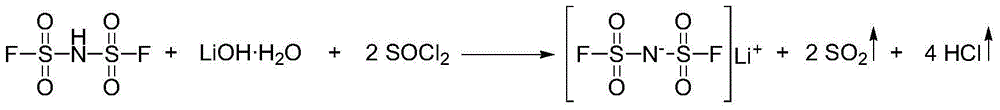
[0047] The invention provides a kind of preparation method of bisfluorosulfonyl imide lithium salt (LiFSI), comprising the following steps:
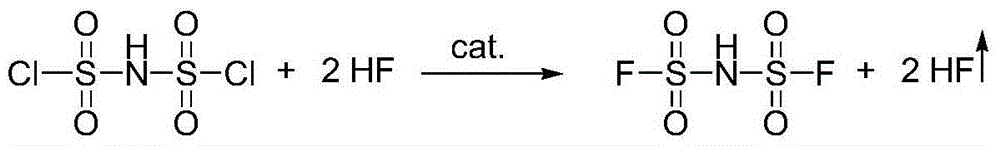
[0048] (1) Fluorination reaction: The intermediate bisfluorosulfonimide (HFSI) is synthesized from bischlorosulfonimide (HClSI) and hydrogen fluoride (HF) under the action of a catalyst. The reaction equation is as follows:
[0049]
[0050] In the step 1, the specific method of the fluorination reaction is: placing HClSI and the catalyst in the reaction device, and feeding HF gas to carry out the reaction.
[0051] In said step 1, the catalyst is preferably a Lewis acid, more preferably SbCl 5 , TiCl 4 , SnCl 4 , MoCl 5 The combination of one or more of them, the molar ratio of HClSI to catalyst is preferably 1:0.05‰~1:1‰, more preferably 1:0.1‰~1:0.5‰.
[0052] In the step 1, the molar ratio of HClSI to HF is preferably 1:1.4-1:4, more preferably 1:1.7-1:2.
[0053] In the step 1, the reaction temperature of the reaction system...
Embodiment 1
[0073] In the 1000mL reaction bottle, add HClSI 1235g, SbCl 5 0.5g, heat up to 100-105°C, slowly pass in about 230g of HF gas under stirring, cool down to room temperature after 20 hours of reaction, blow nitrogen for 15 hours, and obtain about 900g of crude product, short-distilled to obtain 865.6g of product, yield 82.8% .
Embodiment 2
[0075] In the 1000mL reaction bottle, add HClSI 1230g, MoCl 5 0.47g, heat up to 100-105°C, slowly pass in about 216g of HF gas under stirring, cool down to room temperature after 22 hours of reaction, blow nitrogen for 15 hours, and obtain about 896g of crude product, short-distilled to obtain 864.8g of product, yield 83.1% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com