Novel method for preparing triglycidyl isocyanurate
A technology of glycidyl ester and isocyanuric acid tri, which is applied in the direction of organic chemistry and coating, can solve the problems of affecting the electrical performance of TGIC, low yield, and high recycling cost, so as to solve the problem of sewage treatment, reduce production cost, Guaranteed productivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
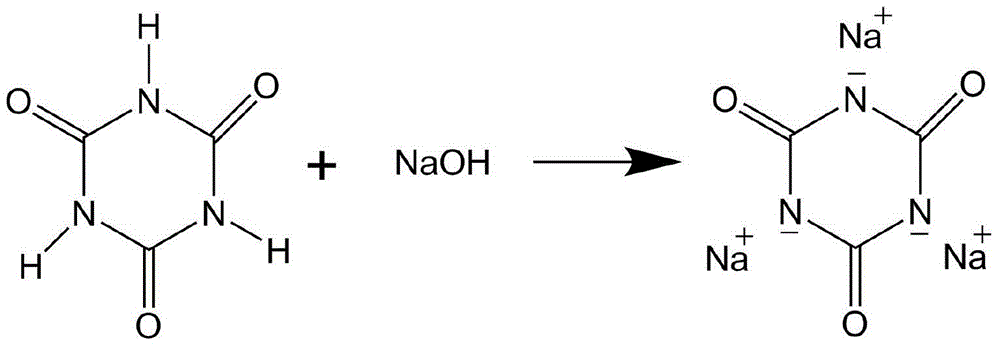
[0015] 10 g of isocyanuric acid was added into the four-necked flask, and at the same time, 50% sodium hydroxide solution was slowly added dropwise, and the reaction temperature was maintained at 60°C. During the reaction, the pH value of the reaction system was maintained at 7.5. After mechanical stirring for 0.5 hours, the water was distilled off under reduced pressure, and the remaining liquid was filtered to obtain a white solid, which was dried in an oven for 12 hours and then used for later use. Through infrared detection and liquid phase detection, it was proved that isocyanuric acid has been combined with hydrogen The sodium oxide reacted completely, and the yield of the reaction was 95%.
[0016] Add the dried trisodium isocyanurate (moisture content≤0.5%) and excess epichlorohydrin (mass ratio: 18:1) into the flask at the same time, and add the phase transfer catalyst benzyl trimethyl chloride ammonium chloride. The reaction is carried out under the condition of an...
Embodiment 2
[0019] 10 g of isocyanuric acid was added into the four-necked flask, and at the same time, 50% sodium hydroxide solution was slowly added dropwise, and the reaction temperature was maintained at 55°C. During the reaction, the pH value of the reaction system was maintained at 7.5. After mechanical stirring for 1 hour, the water was distilled off by vacuum distillation, and the remaining liquid was filtered to obtain a white solid, which was dried in an oven for 12 hours and then used for later use. Through infrared detection and liquid phase detection, it was proved that isocyanuric acid had been combined with hydrogen The sodium oxide reacts completely, and the yield of the reaction is above 98%.
[0020] Add the dried trisodium isocyanurate (moisture content ≤ 1.0%) and excess epichlorohydrin (mass ratio: 15:1) into the flask at the same time, and add the phase transfer catalyst benzyl triethyl chloride ammonium chloride. The reaction is carried out under the condition of ...
Embodiment 3
[0023] 10 g of isocyanuric acid was added into the four-necked flask, and at the same time, 50% sodium hydroxide solution was slowly added dropwise, and the reaction temperature was maintained at 50°C. During the reaction, the pH value of the reaction system was maintained at 8.2. After 2 hours of mechanical stirring, the water was distilled off under reduced pressure, and the remaining liquid was filtered to obtain a white solid, which was dried in an oven for 12 hours and then used for later use. Through infrared detection and liquid phase detection, it was proved that isocyanuric acid had been combined with hydrogen The sodium oxide reacts completely, and the yield of the reaction is above 98%.
[0024] Add the dried trisodium isocyanurate (moisture content ≤ 2.0%) and excess epichlorohydrin (mass ratio: 16:1) into the flask at the same time, and add the phase transfer catalyst benzyl triethyl chloride ammonium chloride. The reaction is carried out under the condition of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy equivalent | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com