A genetically engineered Staphylococcus aureus phage lyase and its preparation method and application
A phage lysing enzyme and Staphylococcus technology, applied in the field of Staphylococcus aureus phage lysing enzyme and its preparation, can solve problems such as difficult to produce resistant bacteria, antibiotic residues in milk, difficult to control Staphylococcus aureus, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Staphylococcus aureus YZ-12, YZ-15, YZ-16, YZ-19, YZ-34, YZ-42, YZ-49, YZ-56, XG-9, XG-25, SF- 16. SF-22, SF-37, SF-46, SF-54, SF-56, A14, A16, X5-1, X12, M-9, S4 and S6 were all isolated, identified and preserved in this laboratory. Typhimurium Salmonella (HNER178, Salmonella Typhimurium is also isolated and preserved in this laboratory, and Staphylococcus aureus (ATCC25923), Salmonella enteritidis (ATCC13076, SalmonellaEnteritidis) and Escherichia coli (ATCC25922) are all purchased from ATCC. Escherichia coli TransB (DE3) is the Commonly used strains, pET32b(+) is a commonly used plasmid in this field.Example 1: Cloning of lyase LysS1 gene (lysS1) and construction of expression vector.
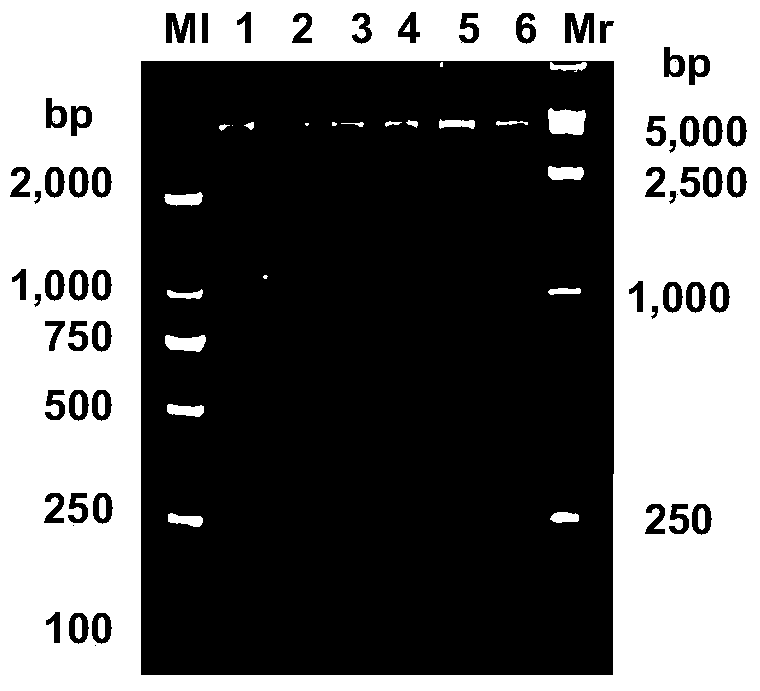
[0045] a. First design the lyase LysS1 gene, which is named as the lyase LysS1 gene, and its nucleotide sequence is SeqID NO.1;
[0046] b. The lyase LysS1 gene was synthesized by Shanghai Sangong and subcloned to obtain a recombinant expression plasmid and named pET32b-LysS1; the ne...
Embodiment 2
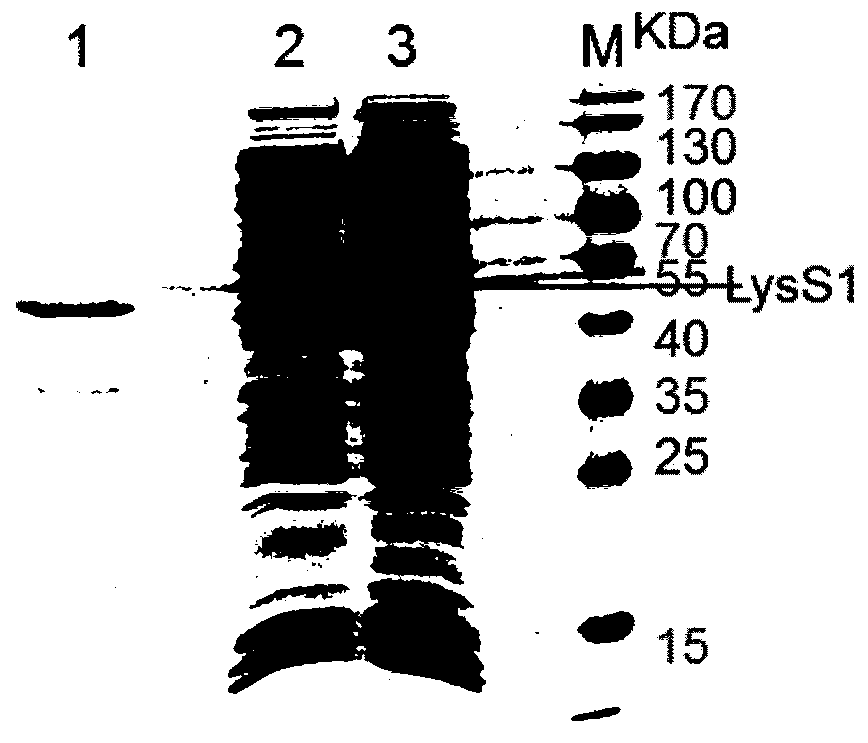
[0049] Example 2: Induced expression and purification of lyase LysS1 protein
[0050] Inoculate the recombinant strain TransB (pET32b-LysS1) into LB culture medium containing ampicillin (50 μg / mL), shake overnight at 37°C; the next day, transfer to 100mL LB medium at a ratio of 1:100, and inoculate at 37°C Shake culture to OD 600 When the value is about 0.5, add IPTG to a final concentration of 1.0mmol / L, and induce at 26°C for 20h. Collect the bacteria, disrupt the cells by ultrasonic, centrifuge at 10,000 rpm / min at 4°C for 10 min, collect the supernatant, filter the supernatant through a 0.22 μm filter membrane, and analyze the protein expression in the lysed supernatant by SDS-PAGE. The filtered lysed supernatant was purified with a His affinity chromatography nickel column (GE Healthcare, Sweden), specifically according to the instructions of the kit. The obtained protein was named LysS1, and the purified LysS1 product was detoxified (≤0.01 EU / μg endotoxin) through a de...
Embodiment 3
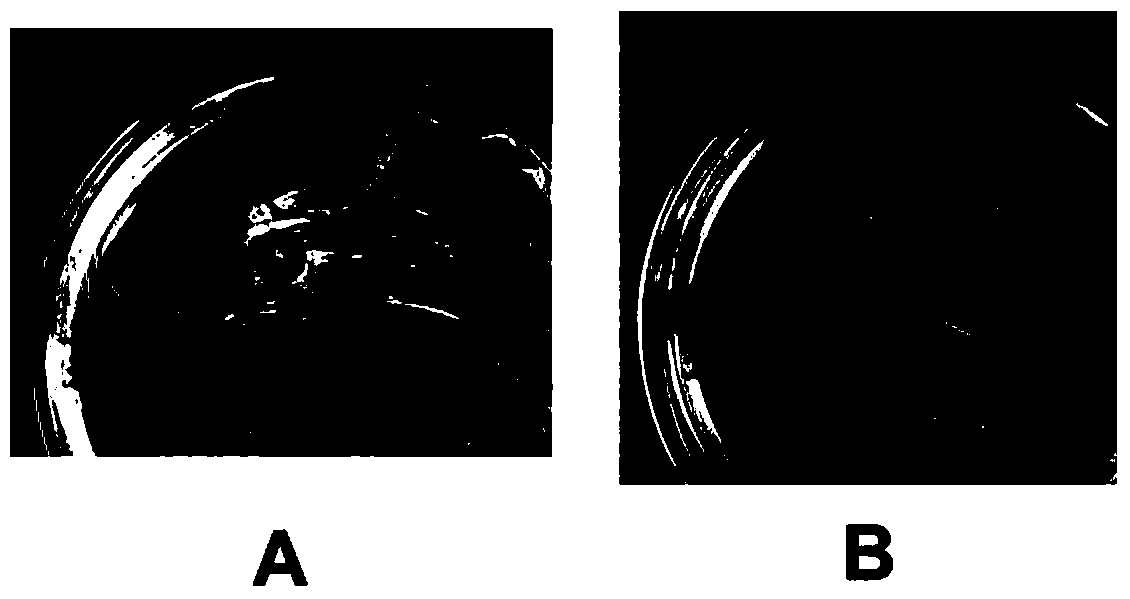
[0052] Embodiment 3: zymogram analysis of lyase LysS1
[0053] Divide the LB (containing 1.2% agarose) plate into several areas, absorb the overnight culture of different host bacteria: Staphylococcus aureus YZ-12, YZ-15, YZ-16, YZ-19, YZ-34, YZ- 42, YZ-49, YZ-56, XG-9, XG-25, SF-16, SF-22, SF-37, SF-46, SF-54, SF-56, A14, A16, X5-1, X12, M-9, S4, S6, all of the above are isolated, identified and preserved in this laboratory and Staphylococcus aureus (ATCC25923), 0.1mL is dropped on the center of the TSB plate, spread the bacterial solution evenly, and incubate at 37°C for 1 hour; At the same time, 0.1 mL of Salmonella typhimurium (HNER178, Salmonella Typhimurium (separated and preserved in our laboratory), Salmonella enteritidis (ATCC13076, Salmonella Enteritidis) and Escherichia coli (ATCC25922) were dropped on the TSB plate and spread evenly; The vector plasmid (pET32b) induced expression product and the buffer solution where the enzyme was located were 10 μL each, and wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com