Protein refolding method using metal ion chelate affinity chromatography column as solid phase carrier
A technology of metal ions and solid phase carriers, applied in the field of protein renaturation, to achieve the effects of shortening the time of protein renaturation, efficient protein renaturation, and avoiding mutual aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
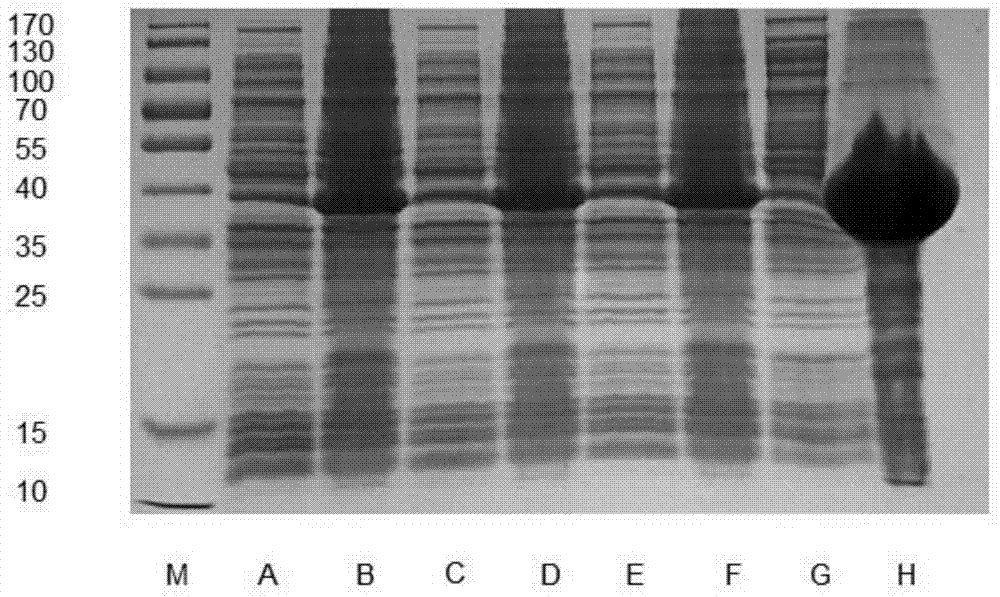
[0017] Example 1 Nickel ion metal chelate affinity chromatography column solid-phase protein refolding
[0018] (1) The inclusion body of SUMO-FGF19 was lysed and centrifuged to collect the precipitate, namely SUMO-FGF19;
[0019] (2) Suspend the above precipitate with denaturing buffer (25mM tris(hydroxymethyl)aminomethane-hydrochloric acid buffer (Tris-HCl), pH=8.0, 150mM NaCl, 8M urea, 50mM β-mercaptoethanol), and in Stir at room temperature for 1 hour. Centrifuge at 18,200 rpm for 30 minutes at 4°C, take the supernatant, and detect the protein concentration with the Coomassie Brilliant Blue G250 method;
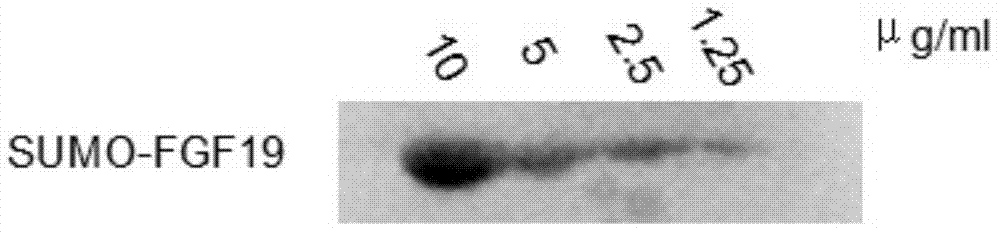
[0020] (3) Pre-equilibrate the nickel metal ion chelation affinity chromatography column with the above-mentioned denaturing buffer. Load 20 ml of 4 mg / mL denatured SUMO-FGF19 solution on a nickel metal ion chelate affinity column at a flow rate of 1.5 ml per minute; after the loading is complete, wash with denaturing buffer to remove proteins that are not bound to the ...
Embodiment 2
[0021] Example 2 Nickel ion metal chelate affinity chromatography solid-phase protein refolding
[0022] (1) The inclusion body of SUMO-FGF19 was lysed and centrifuged to collect the precipitate, namely SUMO-FGF19;
[0023] (2) Suspend the above precipitate with denaturing buffer (25mM Tris-HCl, pH=8.0, 150mM NaCl, 8M urea, 50mM β-mercaptoethanol), and stir at room temperature for 1 hour. Centrifuge at 18,200 rpm for 30 minutes at 4°C, take the supernatant, and detect the protein concentration with the Coomassie Brilliant Blue G250 method;
[0024](3) Pre-equilibrate the nickel metal ion chelation affinity chromatography column with the above-mentioned denaturing buffer. Load 20 ml of 4 mg / mL denatured SUMO-FGF19 solution on a nickel metal ion chelate affinity column at a flow rate of 1.5 ml per minute; after loading, wash with denaturing buffer to remove unbound protein , until the signal balance; within 12 hours, at a flow rate of 0.1 ml per minute, the mobile phase was r...
Embodiment 3
[0033] Example 3 Cobalt ion metal chelate affinity chromatography solid-phase protein refolding
[0034] (1) The inclusion body of SUMO-FGF19 was lysed and centrifuged to collect the precipitate, namely SUMO-FGF19;
[0035] (2) Suspend the above precipitate with denaturing buffer (25mM Tris-HCl, pH=8.0, 150mM NaCl, 6M guanidine hydrochloride, 50mM β-mercaptoethanol), and stir at room temperature for 1 hour. Centrifuge at 18,200 rpm for 30 minutes at 4°C, take the supernatant, and detect the protein concentration with the Coomassie Brilliant Blue G250 method;
[0036] (3) Pre-equilibrate the cobalt ion metal chelate affinity chromatography column with the above-mentioned denaturing buffer. Load 20 ml of 4 mg / mL denatured SUMO-FGF19 solution on the cobalt ion metal chelate affinity column at a flow rate of 1.5 ml per minute; after the loading is complete, wash with denaturing buffer to remove proteins that are not bound to the column , until the signal is balanced; within 8 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com