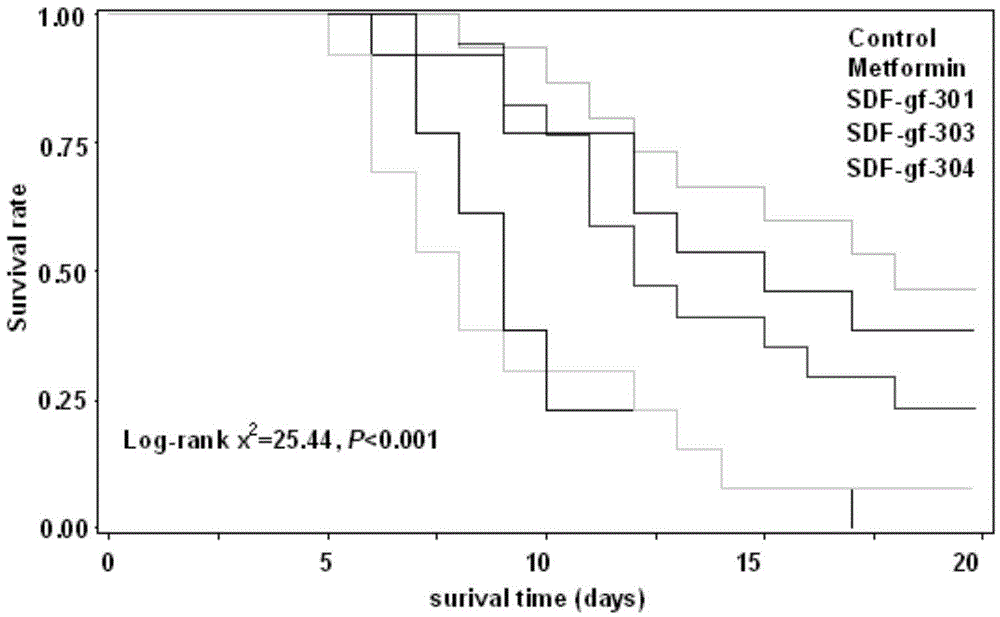
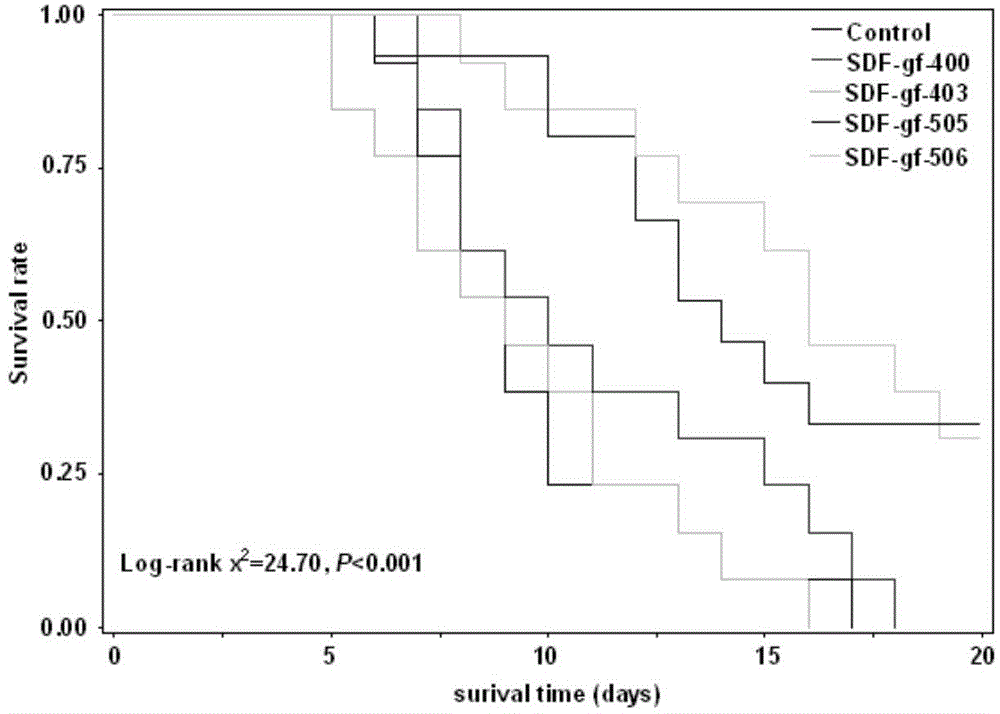
Bis-biguanide, preparation method thereof and application of bis-biguanide
A compound and biguanide technology, applied in the field of medicine, can solve the problems of high cost, lack, curative effect and safety of drugs, etc., to reduce hematopoietic function damage, increase blood cell level, good in vitro activity and animal treatment effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 (synthesis of SDF-gf-106)
[0062] In a 250ml round bottom bottle, add metformin 2g (12mmol), anhydrous acetone 200ml, sodium iodide 3.4g (24mmol), ice bath, stir, add dropwise bromooctane 2.31g (12mmol) and anhydrous acetone 10ml mixed solution , magnetic stirring, thin layer chromatography (TLC) tracking reaction (developing solvent: dichloromethane: methanol: triethylamine = 4.3:0.7:3dr). Reaction at room temperature for 32 hours, post-treatment.
[0063] The reaction solution was filtered, and the filtrate was evaporated to dryness to obtain 2.2 g of an oily substance. The oil was separated with 200-300 mesh silica gel, mobile phase dichloromethane:methanol gradient to obtain 0.55 g of the target product. Yield 19%. 1H NMR(600MHz,DMSO)δ6.86(s,4H),3.04(dd,J=13.4,6.5Hz,2H),2.92(s,6H),1.46(m,2H),1.26(s,10H) , 0.86 (t, J = 7.0 Hz, 3H). HRMS [M+H] 242.41.
Embodiment 2
[0064] Embodiment 2 (synthesis of SDF-gf-111)
[0065] In a 250ml round bottom bottle, add metformin 2g (12mmol), anhydrous DMF 100ml, ice bath, stir, add sodium iodide 3.4g (24mmol), then add dropwise 4-methylbenzyl bromide 2.22g / 1.68ml (12mmol ) and anhydrous MDF15ml mixed solution, magnetic stirring, thin layer chromatography (TLC) tracking reaction (developing solvent: dichloromethane: methanol: triethylamine=4.3:0.7:3dr). Almost complete reaction to metformin hydrochloride. Reaction at room temperature for 15 hours, post-treatment.
[0066] Filter and wash with a little DMF. The filtrate was evaporated to dryness, dichloromethane was added and stirred to obtain about 1.9 g of solid. The solid was recrystallized from acetonitrile to obtain 1.28 g of the target pure product. Yield 45%. 1 H NMR (600MHz, DMSO) δ7.17(d, J=7.9Hz, 2H), 7.03(d, J=7.9Hz, 2H), 5.22(s, 3H), 4.12(s, 2H), 3.34(s , 1H), 2.77(s, 6H), 2.25(s, 3H). HRMS [M+H] 234.37.
Embodiment 3
[0067] Embodiment 3 (synthesis of SDF-gf-117)
[0068] In a 100ml round bottom bottle, add metformin hydrochloride 2g (12mmol), dichloromethane 25ml, ice bath, add 25% sodium hydroxide 10ml, stir, add 4-isopropylbenzyl bromide 2.56g / 2.00ml ( 12mmol), potassium iodide 3 pellets, tetrabutylammonium bromide 0.1g, magnetic stirring, thin layer chromatography (TLC) tracking reaction (developing solvent: dichloromethane: methanol: triethylamine = 4.3:0.7:3dr). A little colorless solid was seen in the reaction flask. Almost complete reaction to metformin hydrochloride. A total of 35 hours at room temperature, post-processing.
[0069] After filtration, 0.1 g of solid was obtained. The filtrate was transferred to a separatory funnel, and the organic layer and the water layer were separated. The water layer was extracted three times with 10 ml of dichloromethane, the organic layers were combined, and the dichloromethane was evaporated to obtain 2.6 g of an oily substance. The solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com