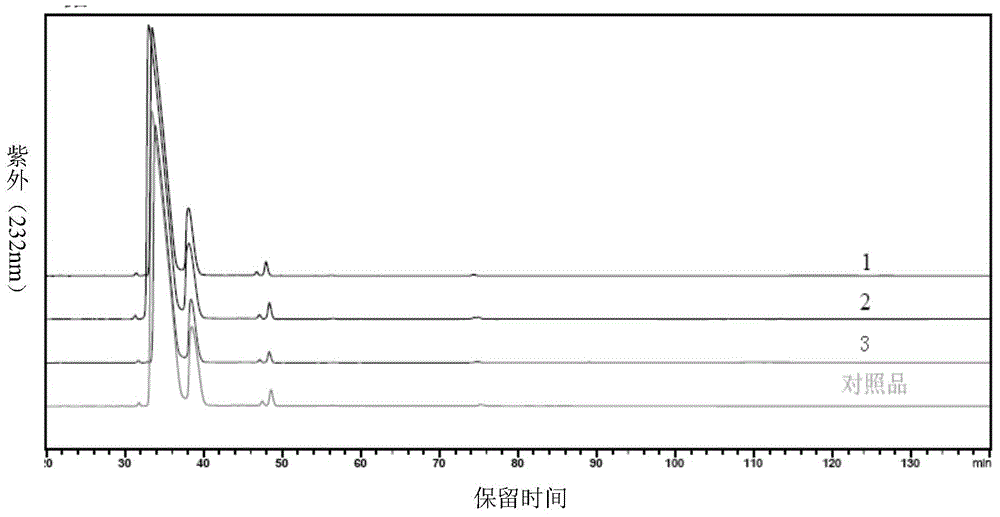
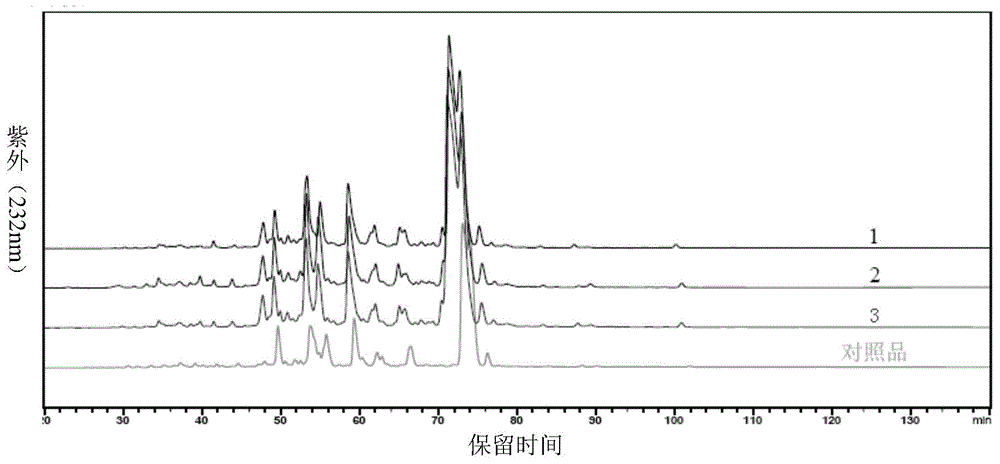
High-purity nadroparin calcium
A nadroparin calcium, high-purity technology, applied in the field of heparin drugs, can solve the problems of less impurities, low quality standard of low molecular weight heparin calcium, high anti-Xa factor/anti-IIa factor titer ratio, and achieve the effect of reducing the burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
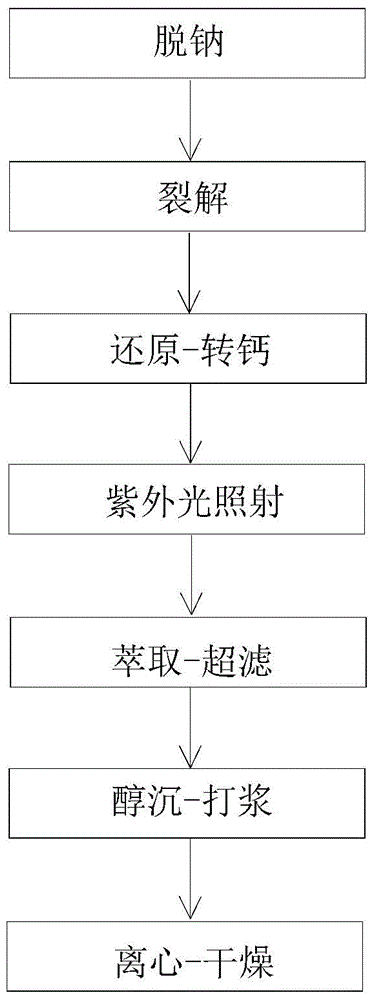
[0035] This embodiment provides a kind of high-purity nadroparin calcium, and the specific steps for preparing the high-purity nadroparin calcium are as follows:
[0036] (1) Desalination
[0037] Dissolve 1 kg of heparin sodium with 8 kg of water to form a heparin sodium solution with a mass percent concentration of 11%, then add a strong acid cation exchange resin to the heparin sodium solution, adjust the pH of the heparin sodium solution to 2.03 by the strong acid cation exchange resin, and then Filtration was performed using a 800-mesh filter cloth to obtain a filtrate.
[0038] (2) cracking
[0039] Add a sodium nitrite solution with a mass percentage concentration of 21.2% to the above filtrate (21.5 g of sodium nitrite is dissolved in 80 mL of water, and all the prepared sodium nitrite solution is put into the filtrate), and then stirred at room temperature for 4 h to obtain a reaction liquid.
[0040] (3) reduction-calcification
Embodiment 2
[0055] This embodiment provides a kind of high-purity nadroparin calcium, and the specific steps for preparing the high-purity nadroparin calcium are as follows:
[0056] (1) Desalination
[0057] Dissolve 1 kg of heparin sodium with 10 kg of water to form a heparin sodium solution with a mass percent concentration of 9%, then add a strong acid cation exchange resin to the heparin sodium solution, adjust the pH of the heparin sodium solution to 2.5 by the strong acid cation exchange resin, and then Filtration was performed using a 800-mesh filter cloth to obtain a filtrate.
[0058] (2) cracking
[0059] Add a sodium nitrite solution with a mass percent concentration of 22.6% to the above filtrate (29.2 g of sodium nitrite is dissolved in 100 mL of water, and all the prepared sodium nitrite solution is put into the filtrate), and then stirred at room temperature for 1 h to obtain a reaction liquid.
[0060] (3) reduction-calcification
Embodiment 3
[0075] This embodiment provides a kind of high-purity nadroparin calcium, and the specific steps for preparing the high-purity nadroparin calcium are as follows:
[0076] (1) Desalination
[0077] Dissolve 3kg heparin sodium with 27kg water to form a heparin sodium solution with a mass percentage concentration of 10%, then add a strong acid type cation exchange resin to the heparin sodium solution, adjust the pH of the heparin sodium solution to 2.4 by the strong acid type cation exchange resin, and then Filtration was performed using a 800-mesh filter cloth to obtain a filtrate.
[0078] (2) cracking
[0079] Add a sodium nitrite solution with a mass percent concentration of 20.2% to the above filtrate (75.8 g of sodium nitrite is dissolved in 300 mL of water, and all the prepared sodium nitrite solution is put into the filtrate), and then stirred at room temperature for 2 h to obtain a reaction liquid.
[0080] (3) reduction-calcification
[0081] Calcium oxide was added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Quality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com