Preparation of iron, cerium and manganese catalyst for eliminating low-concentration nitric oxide at normal temperature
A technology of iron, cerium and manganese catalysts and nitric oxide, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
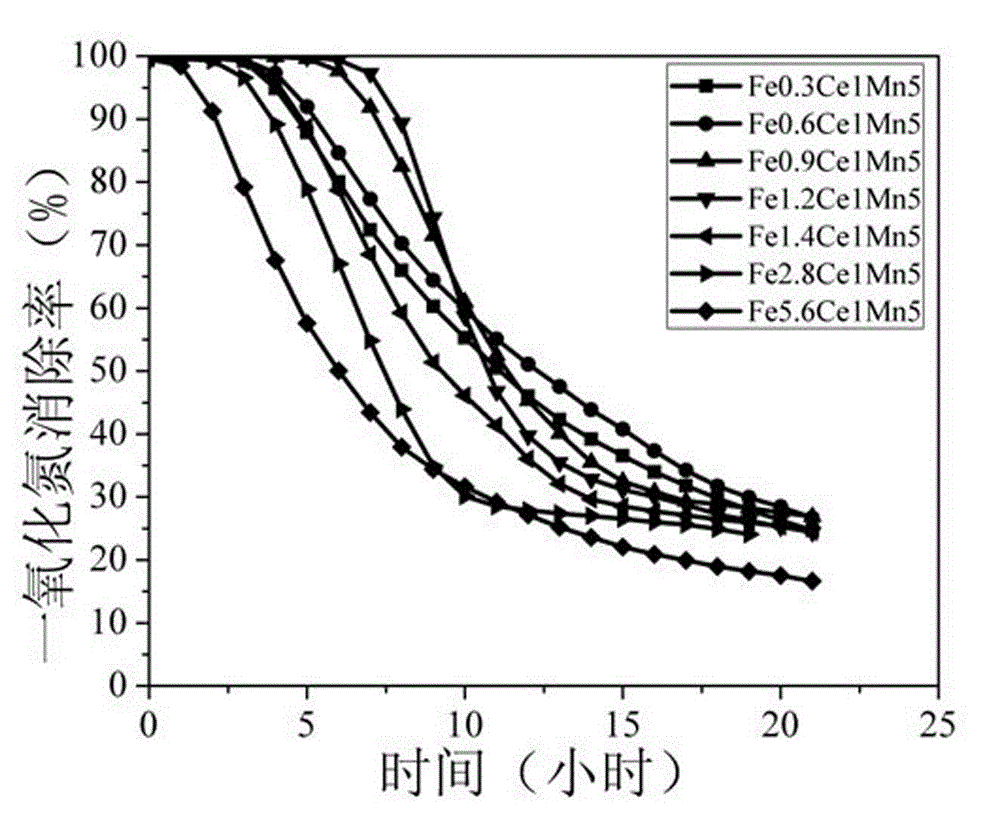
[0015] Weigh 0.42 g of ferrous sulfate, 2.17 g of cerium nitrate, and 4.95 g of manganese chloride in 40 mL of water, stir evenly at room temperature, add dropwise 15 mL of 2.61 mol / L oxalic acid aqueous solution, continue stirring for 1.5 hours, and then filter and wash. Dry in an oven at 70°C for 12 hours, and then bake in a muffle furnace at 300°C for 3 hours to obtain Fe0.3Ce1Mn5, a specific surface area of 87 m 2 / g, the pore size is 7.0 nm.
[0016] Weigh 0.200 g of Fe0.3Ce1Mn5 catalyst and place it in a U-shaped reaction tube with an outer diameter of Φ6 and an inner diameter of Φ4, then put it in a water bath at 25°C, feed 10 ppm NO reaction gas, and the rest is air, with a space velocity of 110,000 mL g -1 h -1 , the highest elimination rate of NO was about 100%, and the elimination rate remained unchanged within 3.7 h.
Embodiment 2
[0018] Weigh 0.83 g of ferrous sulfate, 2.17 g of cerium nitrate, and 4.95 g of manganese chloride in 40 mL of water, stir evenly at room temperature, add dropwise 15 mL of 2.72 mol / L oxalic acid aqueous solution, continue stirring for 1.5 hours, and then filter and wash. Dry in an oven at 70°C for 12 hours, and then bake in a muffle furnace at 300°C for 3 hours to obtain Fe0.6Ce1Mn5, a specific surface area of 91 m 2 / g, the pore size is 6.8 nm.
[0019] Weigh 0.200 g of Fe0.6Ce1Mn5 catalyst and place it in a U-shaped reaction tube with an outer diameter of Φ6 and an inner diameter of Φ4, then put it in a water bath at 25°C, feed 10 ppm NO reaction gas, and the rest is air, with a space velocity of 110,000 mL g -1 h -1 , the highest elimination rate of NO was about 100%, and the elimination rate remained unchanged within 4.0 h.
Embodiment 3
[0021] Weigh 1.25 g of ferrous sulfate, 2.17 g of cerium nitrate, and 4.95 g of manganese chloride in 40 mL of water, stir evenly at room temperature, add dropwise 15 mL of 2.84 mol / L oxalic acid aqueous solution, continue stirring for 1.5 hours, and then filter and wash. Dry in an oven at 70°C for 12 hours, and then bake in a muffle furnace at 300°C for 3 hours to obtain Fe0.9Ce1Mn5, a specific surface area of 96m 2 / g, the pore size is 6.4 nm.
[0022] Weigh 0.200 g of Fe0.9Ce1Mn5 catalyst and place it in a U-shaped reaction tube with an outer diameter of Φ6 and an inner diameter of Φ4, then put it in a water bath at 25°C, feed 10 ppm NO reaction gas, and the rest is air, with a space velocity of 110,000 mL g -1 h -1 , the highest elimination rate of NO was about 100%, and the elimination rate remained unchanged within 6.1 h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Specific surface | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com