Enterovirus 71 type epitope as well as antibody and application and vaccine thereof
An antigenic epitope, enterovirus technology, applied in the fields of molecular biology and immunology, to achieve the effect of high neutralization titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
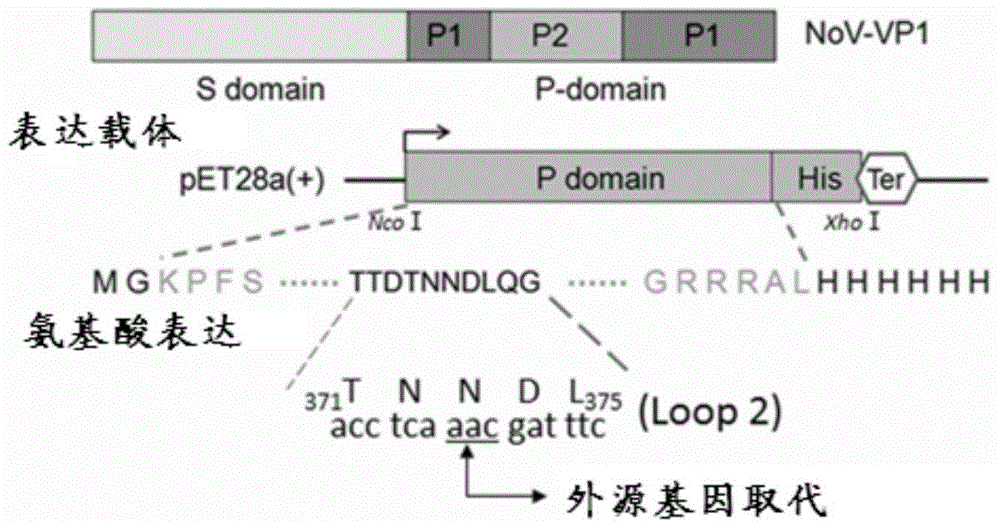
[0063] A fragment of the nucleotide sequence shown in SEQ ID NO: 2 was constructed into the Noru P particle plasmid expression vector PET28a-P domain
[0064] Primers were designed as follows:
[0065]
[0066] GCGGCCGC is the Not I restriction site, CCATGG is the Nco I restriction site, CTCGAG is the Xho I restriction site, and additional GCs are added to avoid frameshift mutations. Obtain the PCR product fragment of the target gene by overlapping PCR, recover the PCR product fragment of the target gene and connect it to the amplified plasmid Peasy-Blunt, transform Escherichia coli, screen positive transformants, extract the plasmid after culture, and treat the plasmid with NcoI and XhoI double enzymes. The original plasmid PET28a-P domain that had been digested with NcoI and XhoI was ligated with T4 ligase to obtain the expression plasmid. The expression plasmid construction process is as follows figure 1 shown.
[0067] The expression plasmid was transformed into expr...
Embodiment 2
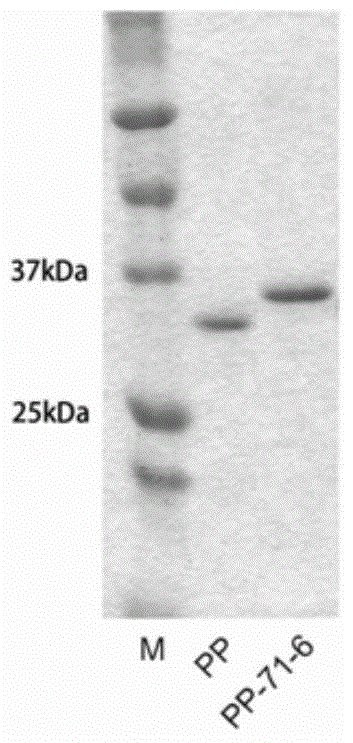
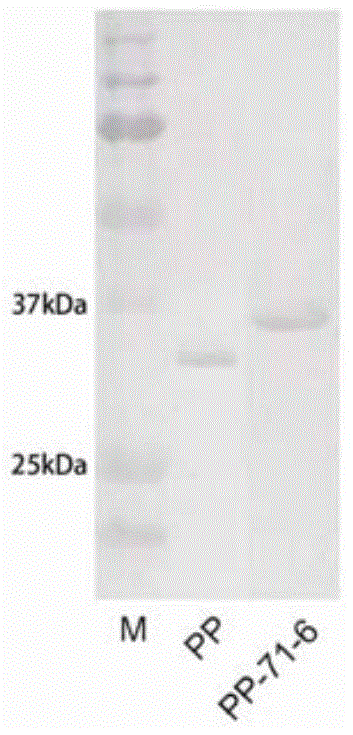
[0071] Take 1 L of antigen-presenting cells induced in Example 1, blow evenly with 80ml of equilibrium solution (20mM Tris, 0.5M NaCl, [pH 8.0]), sonicate for 40min at 4°C, sonicate for 5s and stop for 5s, and keep the precipitate at 16000rpm for 30min. Urea balance solution treatment (8M urea, 20mM Tris, 0.5M NaCl, [pH 8.0]). Stir overnight at 4°C, then keep the supernatant at 10,000rpm for 30min the next day, and present it to the nickel ion affinity layer suction column. The eluent is urea equilibrium solution containing imidazole, and the final elution gradient of the target protein is 200mM imidazole to obtain the fusion protein. Use the same method to purify the control strain expressing the control protein.
[0072] respectively by SDS-PAGE ( Figure 2-a ), western blot( Figure 2-b ), analysis of two-color infrared laser imaging system ( Figure 2-c ) to identify the fusion protein and the control protein, and the two-color infrared laser imaging system quantifies t...
Embodiment 3
[0075] The fusion protein that embodiment 2 makes and AL (OH) 3 The adjuvant is mixed, and the mass ratio of the fusion protein to AL(OH)3 is 25 μg:200 μg / dose to prepare a vaccine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com