Preparation method for ivabradine and pharmaceutical salt thereof
A technology of salt-forming reaction and hydrogen transfer, which is applied in the direction of organic chemistry, can solve the problems that the direct reduction method of borane and alkylsilane cannot be used, and achieve the effects of avoiding equipment requirements, safe operation, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
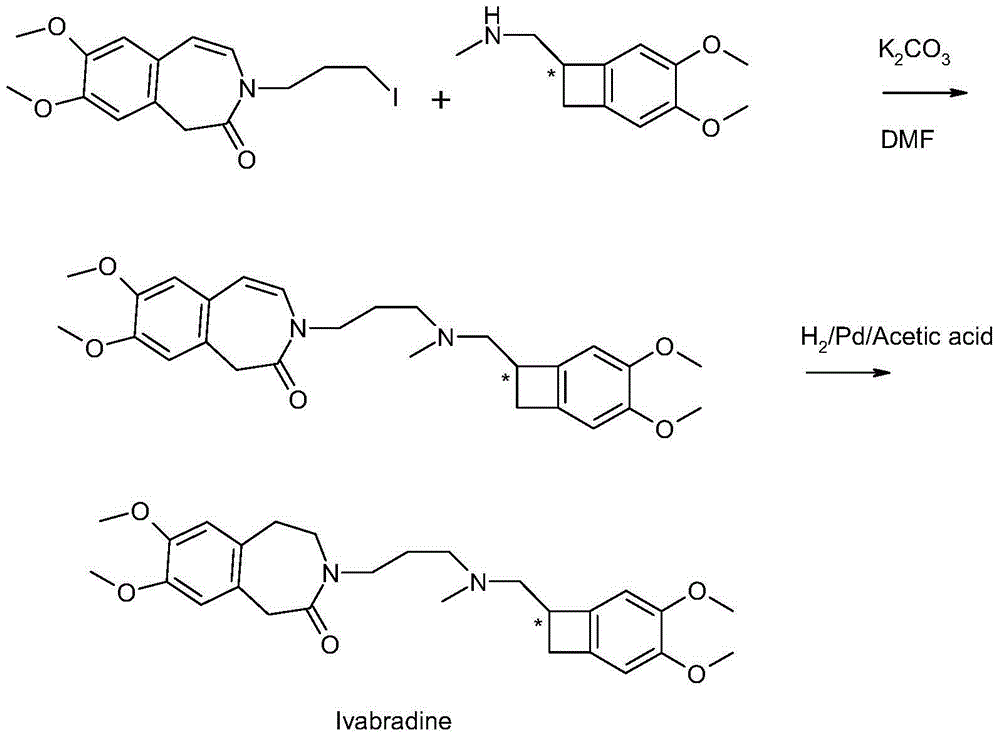
[0022] Example 1 3-3{-[{[(7S)-3,4-dimethoxybenzocyclobutene-7-]methyl}(methyl)amino-propyl]-7,8-di Preparation of Methoxy-1,3-dihydro-2-hydro-3-benzazepin-2-one
[0023] In a 500mL flask, add 25g (0.121mol) (1S)-4,5-dimethoxy-1-[(methylamino)methyl]benzocyclobutane and 250mL DMF, then under nitrogen protection conditions, While stirring, add 45g (0.116mol) 1,3-dihydro-3-(iodopropyl)-7,8-dimethoxy-2H-benzazepin-2-one, 56g powdered potassium carbonate and 65mL DMF, slowly heated to 40-45°C, and incubated for 12 hours. After the reaction was completed, cool to 25°C, add 200ml of toluene and 700ml of water, stir for 10min, and then let stand to separate layers. Continue to add 100mL toluene and 150mL water to the water phase, and then separate the layers again. The organic phases were combined, washed with 100ml of water, and the separated organic phase was spin-dried at 50°C to finally obtain 45g of oily substance 3-3{-[{[(7S)-3,4-dimethoxybenzocyclobutene -7-]methyl}(methyl)...
Embodiment 2
[0024] Example 2 3-3{-[{[(7S)-3,4-dimethoxybenzocyclobutene-7-]methyl}(methyl)amino-propyl]-7,8-di Preparation of Methoxy-1,3,4,5-tetrahydro-2-hydro-3-benzazepin-2-one hydrochloride (formic acid + ammonium formate)
[0025] Under stirring conditions, 46.6g (0.1mol) oily matter, 200ml ethanol, 10g 10%Pd / C (measured in dry state), 27.6g (0.6mol) formic acid and 63g (1mol) Ammonium formate was reacted at room temperature for 12 hours. After the reaction was complete, the catalyst was removed by filtration, and then the formic acid was distilled off under vacuum conditions. To the obtained residue was added 200 mL of acetonitrile, and the pH was adjusted to 3.0˜3.5 by dropwise adding 36% hydrochloric acid. Stir at 0-5° C. for 1 h, filter and dry the product to obtain 40 g of the product, the HPLC purity is 99.8%, the single impurity content is not more than 0.06%, and the yield is 85%.
Embodiment 3
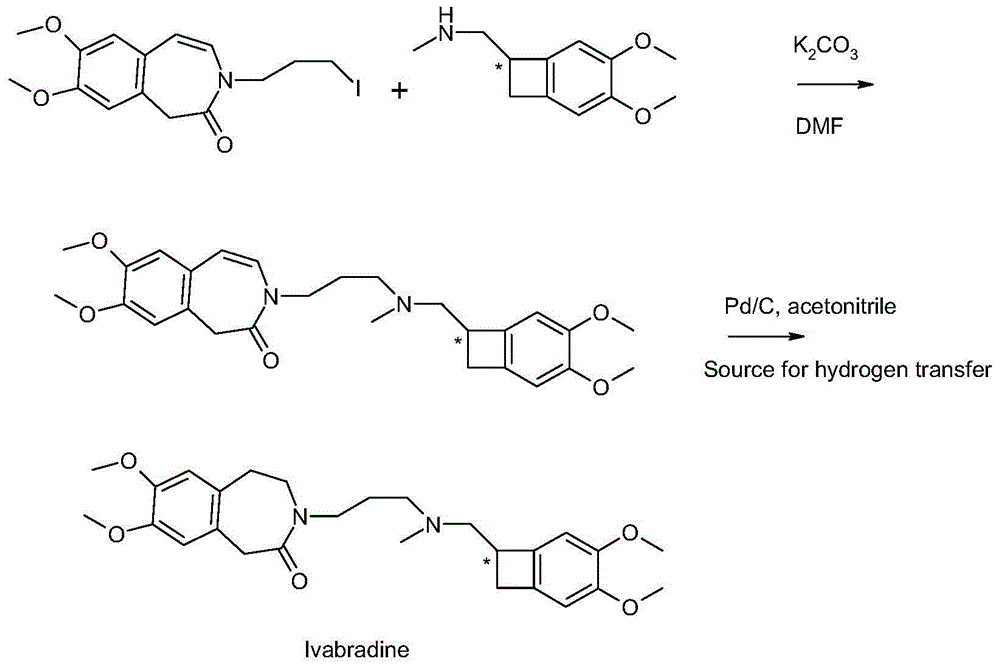
[0026] Example 3 3-3{-[{[(7S)-3,4-dimethoxybenzocyclobutene-7-]methyl}(methyl)amino-propyl]-7,8-di Methoxy--1,3,4,5-tetrahydro-2-hydro-3-benzazepin-2-one hydrochloride} (formic acid and triethylamine)
[0027] Under stirring conditions, 46.6g (0.1mol) oily matter obtained according to the method of Example 1, 200ml acetonitrile, 10g 10%Pd / C (measured in dry state), 30g (0.65mol) formic acid and 27g (0.27mol) ) mixed with triethylamine and then incubated at 50°C for 16h. Remove Pd / C by filtration, distill off volatiles under reduced pressure, then add 200 mL of acetonitrile, and adjust the pH to 3.0-3.5 by adding 36% hydrochloric acid dropwise. Stir at 0-5° C. for 1 h, then filter and dry the product to obtain 45 g of dry product, the HPLC purity is 99.83%, the single impurity content is not more than 0.06%, and the yield is 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com