Cefpodoxime axetil immediate-release preparation and preparation method thereof
A cefpodoxime axetil and preparation technology, applied in the field of medicine, can solve the problems of high equipment requirements, cumbersome production process, and low dissolution rate of preparations, and achieve the effects of avoiding hydrogelation, improving dissolution rate, and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
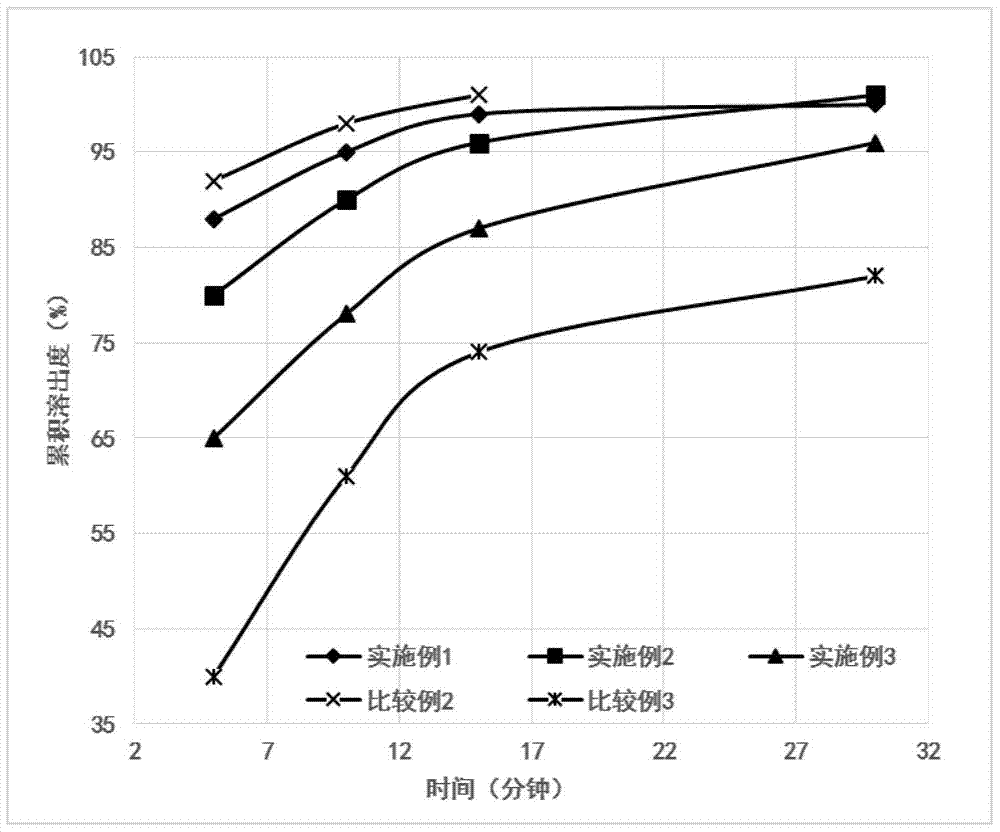
Embodiment 1
[0045] Embodiment 1 (1000 bag amounts)
[0046]
[0047] making process:
[0048] (1) Dissolve the prescribed amount of cefpodoxime axetil and polyoxyethylene hydrogenated castor oil in absolute ethanol to obtain mixture 1.
[0049](2) Put anhydrous lactose, sucrose and carboxymethyl cellulose calcium in a fluidized bed and mix evenly, slowly spray into the mixture 1, granulate, and dry at 30°C in a fluidized bed to obtain the mixture 2.
[0050] (3) Add xanthan gum, citric acid, talcum powder and orange essence to mixture 2, mix them all, and pack them separately.
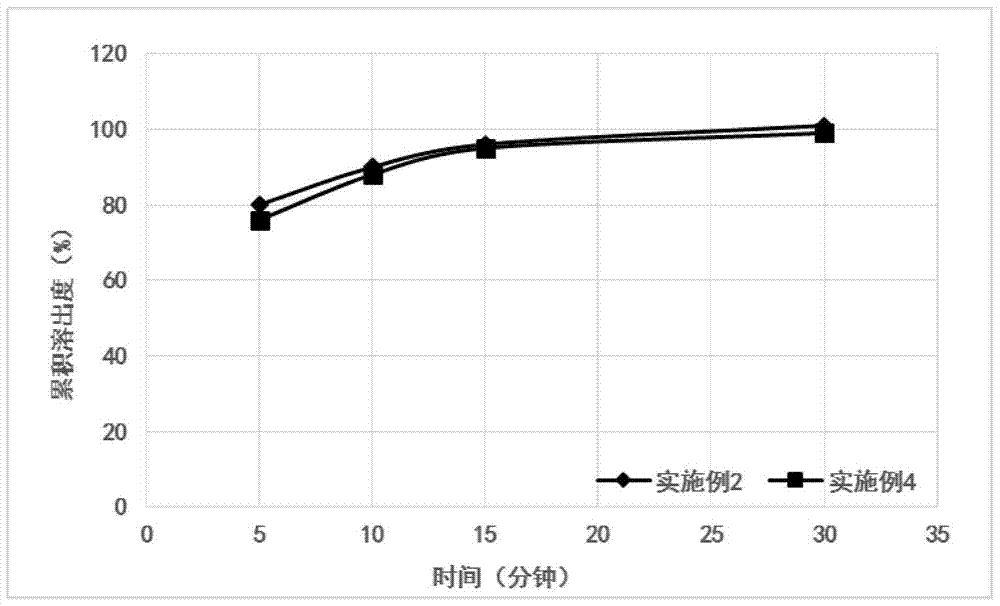
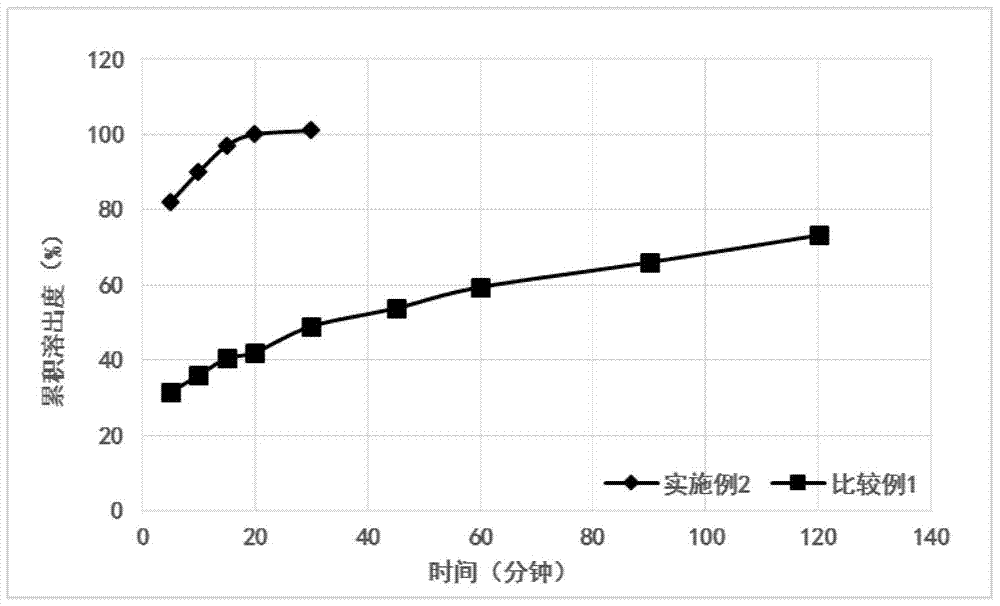
Embodiment 2
[0051] Embodiment 2 (1000 bag amounts)
[0052]
[0053] making process:
[0054] (1) Dissolve the prescribed amount of cefpodoxime axetil and polyoxyethylene hydrogenated castor oil in absolute ethanol to obtain mixture 1.
[0055] (2) Put anhydrous lactose, sucrose and carboxymethyl cellulose calcium in a fluidized bed and mix evenly, slowly spray into the mixture 1, granulate, and dry at 30°C in a fluidized bed to obtain the mixture 2.
[0056] (3) Add xanthan gum, citric acid, talcum powder and orange essence to mixture 2, mix them all, and pack them separately.
Embodiment 3
[0057] Embodiment 3 (1000 bag amounts)
[0058]
[0059] making process:
[0060] (1) Dissolve the prescribed amount of cefpodoxime axetil and polyoxyethylene hydrogenated castor oil in absolute ethanol to obtain mixture 1.
[0061] (2) Put anhydrous lactose, sucrose and carboxymethyl cellulose calcium in a fluidized bed and mix evenly, slowly spray into the mixture 1, granulate, and dry at 30°C in a fluidized bed to obtain the mixture 2.
[0062] (3) Add xanthan gum, citric acid, talcum powder and orange essence to mixture 2, mix them all, and pack them separately.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com